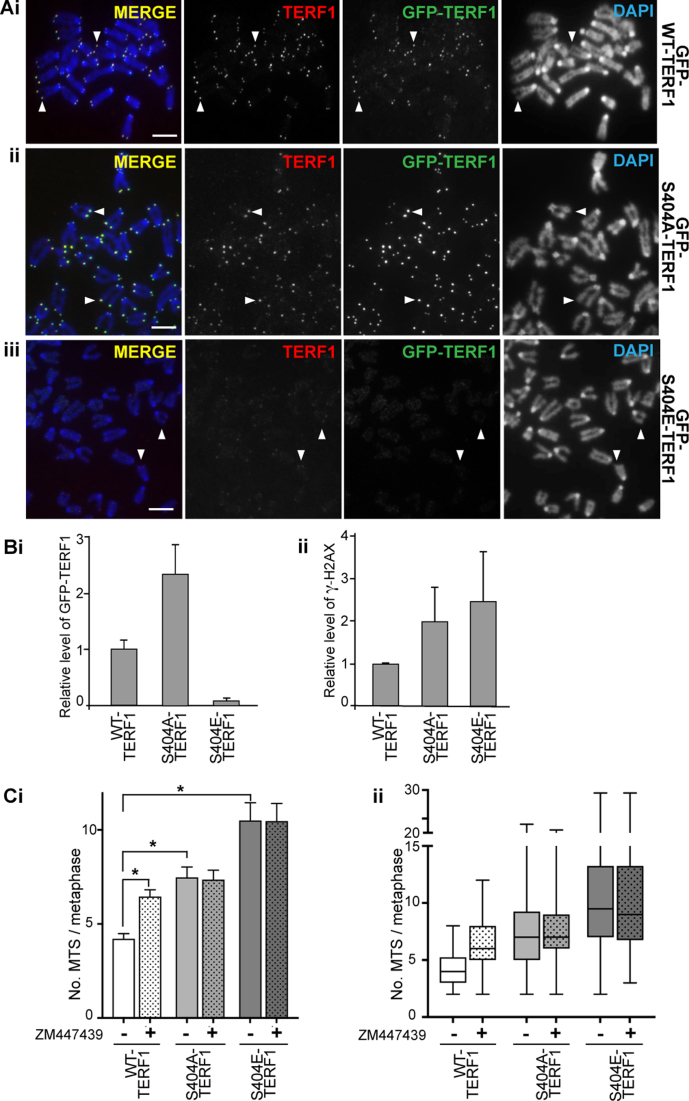
Figure 5.
Phosphorylation of S404-TERF1 controls TERF1 binding to the telomere. (A) Mouse ESCs stably expressing GFP-tagged WT-TERF1 (i), phospho-null TERF1-S404A (ii) and phospho-mimic TERF1-S404E (iii), respectively. Cytospun metaphase chromosomes showed clear binding of GFP-WT-TERF1 (i) and GFP-S404A-TERF1 (ii) at the telomeres (arrowheads). However, GFP-S404E-TERF1 binding could not be detected at the telomeres (arrowheads in iii). In GFP-S404E-TERF1 cells, staining of endogenous TERF1 was also greatly reduced (iii). (B) ChIP/qPCR experiments in GFP-tagged WT-TERF1, S404A-TERF1 and S404E-TERF1 cells. When compared to that of GFP-WT-TERF1, GFP-S404A-TERF1 binding at telomere DNA was increased by 2.3-fold while GFP-S404E-TERF1 showed a greatly reduced binding (∼7% of GFP-WT-TERF1 binding) (i). ChIP/qPCR analyses showing increases in γ-H2AX at telomeres in GFP-S404A and S404E-TERF1 stable cell lines (ii). (C) MTS formation was assessed in GFP-WT-TERF1 cells with and without 24 h of ZM447439 treatment. Both GFP-S404A-TERF1 and GFP-S404E-TERF1 cell lines showed increased MTS formation; increased from 4.2 MTS/metaphase in GFP-WT-TERF1 cells to 7.5 and 10.5 MTS/metaphase in GFP-S404A-TERF1 and GFP-S404E-TERF1 cells, respectively. ZM447439 treatment resulted in increased MTS formation, from 4.2 to 6.5 MTS/metaphase in GFP-WT-TERF1 cells. However, ZM447439 treatment did not result in further increase in MTS formation in GFP-S404A-TERF1 and GFP-S404E-TERF1 cells, respectively. The error bars in column graphs (B and Ci) represent standard error of the mean. Asterisk in (Ci) indicates P-values < 0.001; N > 1300 chromosomes from over 30 mitotic spreads per condition from three biological replicates. Box and whiskers plot shown in (C) represent Q1, Q2 and Q3, with the maximum and minimum values plotted. Scalebars represent 5 μm.