Abstract
Clinical practice guidelines provide both local and global recommendations for the use of iron therapy in the management of anaemia in patients with chronic kidney disease. However, physicians must interpret and adapt these guidelines to meet the specific needs of their individual patients. The recommendations must also be considered in the context of findings from more recently published clinical trials and observational studies.
Keywords: anaemia, CKD, ESRD, ESA, iron
Introduction
Iron therapy, with or without concomitant administration of erythropoiesis-stimulating agents (ESAs), has been used in the management of anaemia in the chronic kidney disease (CKD) population for many years [1]. More recently, the use of iron therapy as a means to delay the need for alternative anaemia management in the pre-dialysis population or to lower the required dosage of ESAs in the haemodialysis (HD) population has come to the fore [2]. The Kidney Disease Improving Global Outcomes (KDIGO) Clinical Practice Guideline for Anemia in Chronic Kidney Disease, published in 2012, provides a set of global recommendations for the use of iron in the treatment of anaemia in CKD [3]. In practice, physicians must adapt these recommendations to best meet the needs of individual patients within the context of their particular health care community. Different countries and institutions may have distinct budgetary, regulatory and practical constraints that impact treatment choices available to physicians and a number of regional organizations have published position statements on the KDIGO guideline or have issued their own clinical practice guidelines [4–9]. The clinical evidence underpinning these guidelines and the areas of continuing debate are discussed in detail in the accompanying chapters by Iain Macdougall and Jeffrey Berns, respectively. The aim of this article is to outline the specific practical considerations relating to the application of these guidelines, highlighting the key decisions facing the nephrologist.
Initiation of iron therapy
The KDIGO guideline states that in anaemic patients with CKD, iron therapy may be required to increase haemoglobin (Hb) levels without the use of ESAs, to boost iron stores prior to initiation of ESA therapy or enhance the response to ESA therapy once initiated or to treat iron deficiency resulting from ESA therapy. Because ESA therapy can only be effective in the presence of sufficient iron to support increased erythropoiesis—iron deficiency is a major cause of ESA hyporesponsiveness in patients with CKD [10]—it is essential that iron deficiency be addressed in patients prior to (or concomitant with) initiation of ESA therapy. This ensures that the lowest possible ESA dose can be used to achieve the desired increase in Hb levels, minimizing possible safety concerns with the use of high ESA doses [11–13].
Iron deficiency can be classified as either absolute, when there is a deficiency in total body iron stores, or functional, when total body iron stores are adequate (or even elevated) but iron release and delivery from internal stores are insufficient to support erythropoiesis. Iron stores are typically assessed through the evaluation of two biomarkers: serum ferritin and transferrin saturation (TSAT). However, it is important to note that serum ferritin levels may be elevated in the presence of inflammation, which is common among patients with CKD. For this reason, serum ferritin may not be an ideal indicator of iron status when considered alone [14]. Furthermore, transferrin synthesis may also be affected by nutritional status. Alternative tests to evaluate iron stores have been proposed (e.g., reticulocyte Hb content and percentage of hypochromic blood cells); however, these have yet to be adopted as part of routine clinical practice [14]. Within this framework, the KDIGO guideline recommends a trial of iron if TSAT is ≤30% and serum ferritin is ≤500 µg/L in patients with CKD [non-dialysis dependent (CKD-ND) and dialysis-dependent (CKD-5D)] who are not on an ESA in whom an increase in Hb concentration is desired without starting an ESA, and in patients on an ESA in whom an increase in Hb levels or a decrease in ESA dose is desired.
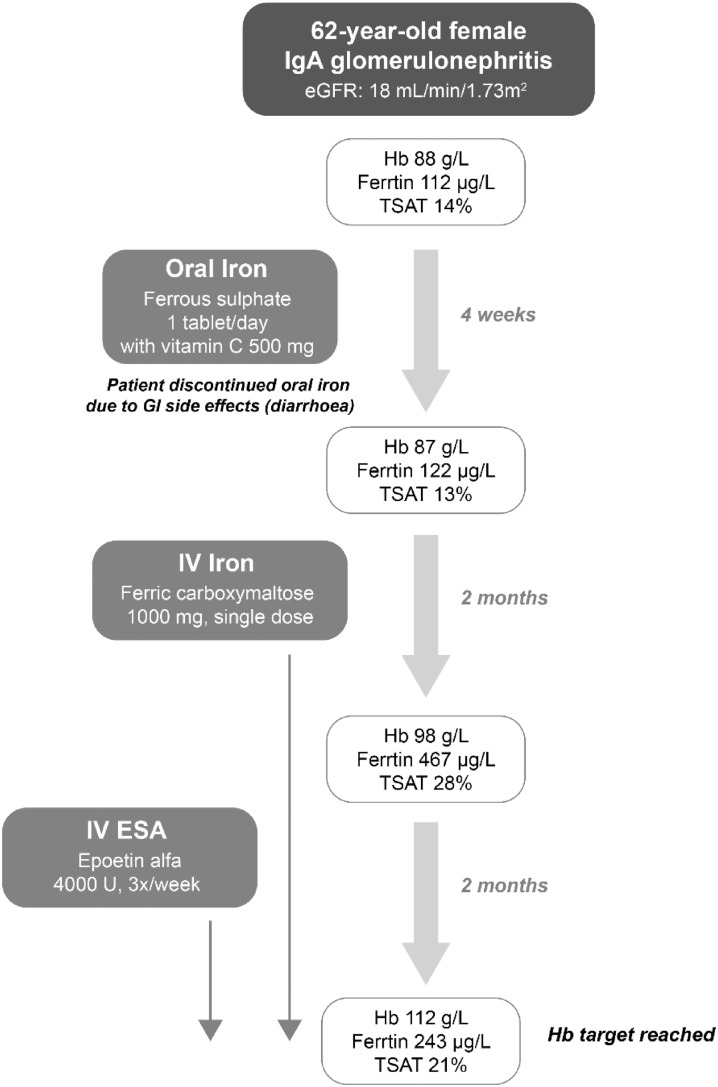
Case study 1 (CKD-ND, Figure 1): A 62-year-old female presenting with IgA glomerulonephritis and eGFR 18 mL/min/1.73 m2 was found to have a Hb level of 88 g/L, serum ferritin of 112 µg/L and TSAT of 14%. C-reactive protein (CRP) was measured at 3.3 mg/L and erythrocyte sedimentation rate (ESR) was not elevated. Based on the evaluation of serum ferritin and TSAT levels, the patient was deemed to be iron deficient and the initiation of iron therapy to correct this deficiency was the first priority for the physician. The initiation of ESA therapy was not appropriate in this case because the patient clearly did not have sufficient iron stores to support erythropoiesis.
Case study 2 (CKD-5D, Figure 2): A 74-year-old male with diabetic nephropathy commenced HD with an Hb level of 104 g/L, serum ferritin of 122 µg/L and TSAT of 16%. The patient was not inflamed, with an ESR of 22 mm/h and CRP of 5.6 mg/L. Prior to initiating HD, the patient had been receiving a subcutaneous ESA; upon starting renal replacement therapy, the route of administration was switched to intravenous (IV) and the patient was treated with 40 µg IV darbepoetin alfa administered weekly at dialysis treatments, in accordance with facility protocols. After 3 months, the patient’s Hb had declined to 94 g/L. In addition, the patient’s serum ferritin had declined to 92 µg/L and TSAT was 15%. In this instance, the patient was already receiving an ESA but treatment had become less effective upon initiation of dialysis and transition from subcutaneous to IV administration. The decline in the patient’s serum ferritin and TSAT levels indicated that the patient had become iron deficient and that further increases in the ESA dose would be inappropriate without first addressing the patient’s iron deficiency.
Fig. 1.
Case study 1: CKD-ND.
Fig. 2.
Case study 2: CKD-5D.
Oral or IV?
Having decided that the initiation of iron therapy is necessary, the clinician must next decide whether oral or IV administration will be best for the individual patient. The KDIGO guideline does not recommend the use of oral iron in CKD-5D patients, but suggests that either oral or IV iron may be considered in CKD-ND patients. Recommendations from other organizations are similar (discussion of this is presented in the accompanying article by Jeffrey Berns). For CKD-ND patients it is suggested that the selection of oral versus IV administration should consider the severity of anaemia, availability of venous access, response to prior therapy, patient adherence and cost.
IV administration of iron has been demonstrated to be more effective than oral administration with respect to the elevation of Hb, ferritin and TSAT levels in patients with CKD-5D and in those with CKD-ND. Patients receiving IV iron have also been shown to achieve target Hb levels more quickly [15–22]. Furthermore, ESA dose requirements are lower in patients treated with IV iron compared with those receiving oral iron [19, 23–26]. A recent meta-analysis that analysed data from seven randomized controlled trials (RCTs) that allowed variable IV iron and ESA dosing showed a 23% reduction in ESA dose attributable to appropriate dosing of IV iron in HD patients [2]. IV rather than oral administration of iron may therefore be more appropriate for CKD patients with more severe anaemia and iron deficiency, as well as for those receiving ESA therapy.
The fact that oral iron does not require administration in the health care setting may make it a convenient option for patients with CKD-ND, who have a lower frequency of health care contact than patients receiving in-centre dialysis. In addition, among CKD-ND patients approaching transition to dialysis, the preservation of the vasculature to allow for the creation of an arteriovenous fistula may be a priority and oral administration may therefore be favoured over IV. However, with the ability to give large dosages of IV iron (up to 1000 mg) at one administration, the number of required IV cannulations can be limited. Oral iron is widely available and inexpensive. However, oral iron therapy imposes a high pill burden on patients, with a typical regimen of 200 mg elemental iron per day as ferrous sulphate requiring the patient to take multiple tablets three times per day. Both the high pill burden and unpleasant side effects associated with oral iron therapy can lead to adherence issues that may ultimately limit efficacy as well as patient quality of life. It should also be considered that uraemia is associated with reduced gastrointestinal absorption of iron, while chronic inflammation and medication interactions (notably, calcium carbonate, which is used by many patients with CKD as a phosphate binder) can also impair gastrointestinal iron uptake, thereby further exacerbating efficacy issues [27]. Recently, two iron-containing phosphate binders have been approved for use in the control of hyperphosphataemia in patients with CKD on dialysis and may also have an effect on the management of anaemia in this patient population [28, 29]. In 2017, iron-containing phosphate binders are not yet approved for use in patients with CKD-ND: a recent study in patients with CKD-ND and iron deficiency anaemia revealed a positive effect on Hb levels and an increase in iron parameters over a 16-week study period; however, long-term safety studies are still needed [30].
Historically there have been concerns about the safety of parenteral iron formulations, particularly high molecular weight iron dextran (no longer available in most countries), which was associated with occasional serious reactions, including anaphylaxis and death, due to immunogenicity of the dextran component [31, 32]. Such reactions are not a significant concern with newer IV iron formulations: the risk of serious reaction is significantly lower for low molecular weight iron dextran than for high molecular weight iron dextran [32] and a retrospective evaluation of >30 million doses of IV iron showed a serious adverse event (SAE) rate of <1 in 200 000 administered doses for non-dextran IV iron preparations [33]. However, a 2013 statement from the European Medicines Agency highlighted the potential for hypersensitivity reactions to IV iron preparations and indicated that patients should be observed for signs and symptoms of such reactions for at least 30 min following administration [34]. Other potential safety issues associated with IV iron use that have been of concern to nephrologists in recent years include the potential for increased oxidative stress, risk for infection and the possibility of iron overload [35]. It has been suggested that the formation of reactive oxygen species as a result of transient increases in labile plasma iron may lead to increased atherogenesis and deleterious cardiovascular (CV) effects, as well as renal damage [36–38]. Iron is an important cofactor for bacterial growth [39, 40] and it has been postulated that the use of IV iron may increase the risk of infection or worsen existing infections [41–44]. Finally, because IV administration of iron bypasses physiological controls over absorption from the gut, it has been suggested that the long-term use of IV iron could result in the deposition of iron in the liver, pancreas and heart, ultimately resulting in organ damage [45–48]. However, several studies have demonstrated increased liver iron content in CKD-5D patients without evidence of accompanying fibrosis [49].
The RCTs and observational data relating to the safety of IV iron are discussed in detail in the article in this supplement by Iain Macdougall; meta-analyses of RCTs assessing IV iron therapy in patients with CKD (both CKD-5D and CKD-ND) have revealed no appreciable differences in the rates of mortality and adverse events (including CV events and infection) for IV and oral iron treatment groups, although it should be noted that the majority of studies evaluated in these analyses had comparatively short follow-up periods [50–52]. Recently published data from clinical trials that were specifically designed to evaluate the longer-term efficacy and safety of IV iron therapy in patients with CKD-ND are equivocal: results from the FIND-CKD study showed no increase in the risk of CV events or infectious episodes among patients receiving IV ferric carboxymaltose compared with those receiving oral iron over a 56-week follow-up period [53, 54]. In contrast, the single-centre REVOKE trial was terminated early due to increased risk of SAEs, CV SAEs and infection in patients receiving IV iron compared with those receiving oral iron [41]. Possible explanations for these apparently conflicting findings include differences in methods of SAE adjudication and reporting as well as the fact that adjustments for baseline differences in patient characteristics were applied in the analysis of data from the REVOKE study [55]. In summary, while the weight of clinical evidence to date generally indicates that the use of IV iron in patients with CKD is both effective and safe, results from ongoing and future trials will be important to confirm long-term safety.
Case study 1 (CKD-ND): In this instance, the patient was not being seen in the health care setting on a frequent basis. Considering this, and in accordance with KDIGO recommendations for CKD-ND patients, a trial of oral iron was initiated with concomitant vitamin C to increase absorption. However, follow-up after 4 weeks revealed that the patient had stopped taking the oral iron due to diarrhoea and there had been no increase in her Hb levels. Although the patient’s serum ferritin level had slightly increased to 122 µg/L, TSAT remained low (13%). Given the patient’s continuing anaemia and inability to tolerate oral iron, switching to IV administration of iron was necessary.
Case study 2 (CKD-5D): Because the patient was receiving in-centre HD, he had a vascular access in place and was being seen in the dialysis unit three times per week. In this case, the administration of IV iron presented no practical difficulties and was in accordance with both facility treatment protocols and clinical practice guidelines.
Selection of IV iron dosing regimen and formulation
Once the clinician has established that initiation of IV iron therapy is the most appropriate course of action, a decision must then be made as to which formulation will be used and how it will be dosed.
The KDIGO guideline provides little guidance on the selection of an IV iron dosing regimen, citing insufficient evidence to support a specific recommendation. However, the guideline does state that the nature of a patient’s clinical encounters may affect the decision. For patients with CKD-ND, who are seen less frequently by a nephrologist and for whom the preservation of potential vascular access sites is important, the use of high-dose, low-frequency IV iron dosing may be preferred. In the recent FIND-CKD study, patients with CKD-ND receiving IV ferric carboxymaltose to target and maintain a serum ferritin level of 100–200 µg/L (200 mg dose every 4 weeks) or 400–600 µg/L (500–1000 mg dose every 4 weeks) were compared with those receiving oral iron therapy (200 mg/day) [53]. The primary endpoint of the study was initiation of other anaemia management (ESA, other iron therapy, transfusion) or occurrence of an Hb trigger (two consecutive Hb values <10 g/dL during Weeks 8–52), which was observed less frequently in the high-ferritin IV ferric carboxymaltose group compared with both the low-ferritin IV ferric carboxymaltose and oral iron groups (in 23.5, 32.2 and 31.8% of patients, respectively; P = 0.026 for high-ferritin IV ferric carboxymaltose versus oral iron). In addition, patients in the high ferritin group had a faster haematological response and were more likely to have an increase in Hb of at least 1 g/dL; there were no differences in AEs and SAEs—including infectious and CV events—between groups. The study authors concluded that the administration of higher doses of IV iron to target higher serum ferritin levels may result in improved anaemia management in patients with CKD-ND. These results were achieved with relatively few FCM injections.
For patients with CKD-5D, an IV iron dosing regimen is likely to be dictated by protocols in place at the dialysis facility. In general, more frequent (weekly or biweekly) administration of lower doses of IV iron during regularly scheduled dialysis sessions is preferred in such patients, although higher doses may be indicated if serum ferritin and TSAT levels fall below thresholds prescribed by the treatment protocol. Observational studies, although limited by the potential risk of confounding, have provided some insights with respect to the efficacy and safety of bolus versus maintenance dosing regimens in CKD-5D patients: bolus dosing has been associated with higher levels of Hb, TSAT and ferritin and the use of lower ESA doses compared with maintenance dosing [56]. However, bolus dosing has also been associated with an increased risk of infection-related hospitalizations [57, 58]. Thus avoidance of bolus dosing in patients with an active infection might be prudent.
The selection of IV iron formulation to be used is primarily dependent on the dosing regimen selected. Currently available IV iron formulations all have a polynuclear iron(III)-oxyhydroxide/oxide iron core stabilized by a carbohydrate shell, but vary with respect to certain physicochemical and pharmacokinetic properties [59–61]. The newer, more stable iron complexes (ferumoxytol, ferric carboxymaltose and iron isomaltoside 1000) do not readily dissociate under physiological conditions, whereas less stable complexes (e.g. iron sucrose and sodium ferric gluconate) must be administered at lower doses or more slowly—as specified by the product label—to avoid overwhelming the binding capacity of transferrin in the serum and potential infusion reactions (Table 1). Thus, for a patient with CKD-ND receiving IV iron on a high-dose, low-frequency schedule, the use of a more stable formulation would be appropriate, whereas in patients with CKD-5D receiving frequent, low-dose IV iron, less stable formulations with lower approved maximum single doses may also be acceptable.
Table 1.
IV iron preparations
| Sodium ferric gluconate | Iron sucrose | Ferric carboxymaltose | Low molecular weight iron dextran | Iron isomaltoside 1000 | Ferumoxytol | |
|---|---|---|---|---|---|---|
| Trade name(s) | Ferrlecita | Venoferb | Injectafer, Ferinjectc | Cosmoferd | Monofere | Ferahemef |
| Formulation | 12.5 mg/mL in 5 mL single-use vial | 20 mg/mL in 2.5 and 5 mL single-use vials and ampoules | 50 mg/mL in 2, 10 and 20 mL single-use vials | 50 mg/mL in 2, 5, and 10 mL single-use ampoules | 100 mg/mL in 1, 2, 5, and 10 mL single-use vials/ampoules | 30 mg/mL in 17 mL as 510 mg single-use vial |
| Maximum single dosage [59, 60] | 125 mg | 200 mg | 1000 mg (up to 200 mg in CKD-HD patients) | 20 mg/kg | 20 mg/kg | 510 mg |
| Minimal administration time [59, 60] | 10–60 min | 10–30 min | 15 min | 4–6 h | 15–30 min | 15 min |
| Test dose required | No | No | No | Yes | No | No |
| FDA black box warning | No | No | No | Yes | NA (available in Europe only) | Yes |
FDA, US Food and Drug Administration; NA, not applicable
Ferrlecit prescribing information: http://products.sanofi.us/ferrlecit/ferrlecit.html (24 April 2017, date last accessed)
Venofer prescribing information: http://www.venofer.com/PDF/Venofer_PI_82015.pdf (24 April 2017, date last accessed)
Ferinject prescribing information: http://www.injectafer.com/pdf/pi.pdf (24 April 2017, date last accessed)
Cosmofer prescribing information: https://www.medicines.org.uk/emc/medicine/14139 (24 April 2017, date last accessed)
Monofer prescribing information: https://www.medicines.org.uk/emc/medicine/23669 (24 April 2017, date last accessed)
Feraheme prescribing information: http://www.feraheme.com/pdfs/Feraheme_Prescribing_Information.pdf (24 April 2017, date last accessed)
Clinical trials in which different IV iron formulations have been compared directly with respect to safety and efficacy have generally involved only small numbers of patients and short follow-up periods. A randomized study in patients with CKD-ND showed a significantly higher risk of serious adverse drug reactions with low molecular weight iron dextran compared with iron sucrose and sodium ferric gluconate [62], while randomized trials comparing iron isomaltoside 1000 [63] and ferumoxytol [64] to iron sucrose showed equivalent efficacy with respect to increases in Hb levels and no significant difference in the frequency of adverse events. An evaluation of ferric carboxymaltose compared with standard medical care (which included no iron, oral iron or IV iron) in both CKD-HD and CKD-ND patients showed lower rates of SAEs in patients receiving a single dose of ferric carboxymaltose compared with both the standard medical care group overall and patients receiving IV iron sucrose or sodium ferric gluconate; there were no significant differences in efficacy with respect to improvement in Hb levels across groups [65].
Beyond considerations of efficacy and safety (which, at least among non-dextran formulations, are largely equivalent), the selection of IV iron formulation is also driven by availability. Some formulations are only available in specific geographic regions [66], while cost and contractual agreements in place at individual health care institutions will also affect which preparations are available to the prescribing physician, as often only a single drug in a particular class will be included on the formulary.
Case study 1 (CKD-ND): For this non-dialysis-dependent patient, the use of ferric carboxymaltose, a newer and more stable formulation that is approved for single-dose administration up to 1000 mg in patients with CKD-ND, avoided the inconvenience of more frequent provider visits. After 2 months, the patient’s serum ferritin and TSAT had increased to 467 µg/L and 28%, respectively, although the patient continued to experience symptoms of anaemia (cold sensitivity, lack of energy, shortage of breath on exertion) and her Hb levels were still <100 g/L. At this point, with sufficient iron stores to support its effective use, an ESA (epoetin alfa) was initiated at a dose of 4000 U, three times per week (50 µg/kg for a patient weighing 80 kg). At the follow-up 2 months later, the patient’s Hb level was 112 g/L and she reported improvement in her symptoms. Thus, after an unsuccessful trial of oral iron, the initiation of IV iron therapy resulted in improved Hb levels, and although this was not sufficient alone, the use of IV iron to ‘top up’ the patient’s iron stores prior to starting ESA therapy ensured that target Hb levels were achieved with the lowest possible ESA dose.
Case study 2 (CKD-5D): The IV iron preparation available for use at the patient’s dialysis facility was iron sucrose. The administration of a weekly 100 mg dose while the patient was on dialysis was practical, in accordance with the facility protocol targeting appropriate ferritin and TSAT values and resulted in an increase in both iron storage and availability markers and Hb levels. Once the target Hb level had been reached, the dose of ESA was reduced while maintenance IV iron therapy was continued. However, after a further 9 months, the patient’s Hb had declined to 94 g/dL. Serum ferritin and TSAT were measured at 363 µg/L and 20%, respectively, and the darbepoetin alfa dose was increased to 60 µg/week IV. In this circumstance—where the ESA dose must be increased to maintain Hb levels in the context of ongoing IV iron use to target specific serum ferritin and TSAT levels—it is critical that the physician consider the possibility of blood loss, as patients receiving IV iron on a protocol-driven schedule will not manifest with iron deficiency. In this case, immunochemical tests for faecal occult blood were positive and colonoscopy revealed the presence of a colonic adenocarcinoma.
Concluding remarks
In summary, while clinical practice guidelines provide general recommendations for the use of iron in the management of anaemia associated with CKD, their application in clinical practice must be tailored to meet the needs of the individual patient. The timing of iron therapy initiation, route of administration, and selection of treatment regimen should take into account a number of factors, including the severity of anaemia and/or treatment goals, CKD stage and dialysis modality, comorbidities and concomitant patient health issues, as well as any relevant practical considerations. The evidence base for the KDIGO guideline recommendations, particularly with respect to the use of IV versus oral iron in patients with CKD-ND, was somewhat limited. However, findings from a number of studies assessing the safety and efficacy of IV iron in this patient population have been published in the intervening years. As a result, IV iron may become the preferred initial treatment option for physicians wanting to increase Hb concentrations or delay alternative anaemia management in patients with CKD-ND.
Acknowledgements
The author wishes to thank Abigail Hunt, PhD, an employee of DaVita Clinical Research, Minneapolis, MN, USA, for medical writing and editorial support in the preparation of this article.
Funding
Funding for medical writing support was provided by Vifor Pharma.
Conflict of interest statement
S.D.R. has received speaker’s fees, honoraria and consultancy fees from manufacturers of ESAs and IV iron, including Amgen, Hoffmann-La Roche, Janssen-Cilag, Novartis, Sandoz and Vifor Pharma.
References
- 1. Kalantar-Zadeh K, Streja E, Miller JE. et al. Intravenous iron versus erythropoiesis-stimulating agents: friends or foes in treating chronic kidney disease anemia? Adv Chronic Kidney Dis 2009; 16: 143–151 [DOI] [PubMed] [Google Scholar]
- 2. Roger SD, Tio M, Park HC. et al. Intravenous iron and erythropoiesis-stimulating agents in haemodialysis: A systematic review and meta-analysis. Nephrology (Carlton) 2016; doi: 10.1111/nep.12940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kidney Disease: Improving Global Outcomes (KDIGO) Anemia Work Group. KDIGO clinical practice guideline for anemia in chronic kidney disease. Kidney Int Suppl 2012; 2: 279–335 [Google Scholar]
- 4. Kliger AS, Foley RN, Goldfarb DS. et al. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for anemia in CKD. Am J Kidney Dis 2013; 62: 849–859 [DOI] [PubMed] [Google Scholar]
- 5. Locatelli F, Barany P, Covic A. et al. Kidney disease: improving global outcomes guidelines on anaemia management in chronic kidney disease: a European renal best practice position statement. Nephrol Dial Transplant 2013; 28: 1346–1359 [DOI] [PubMed] [Google Scholar]
- 6. Macginley R, Walker R, Irving M.. KHA-CARI guideline: use of iron in chronic kidney disease patients. Nephrology (Carlton) 2013; 18: 747–749 [DOI] [PubMed] [Google Scholar]
- 7. Manns BJ, White CT, Madore F. et al. Introduction to the Canadian Society of Nephrology clinical practice guidelines for the management of anemia associated with chronic kidney disease. Kidney Int Suppl 2008; 74(Suppl 110): S1–S3 [DOI] [PubMed] [Google Scholar]
- 8. Ratcliffe LE, Thomas W, Glen J. et al. Diagnosis and management of iron deficiency in CKD: a summary of the NICE guideline recommendations and their rationale. Am J Kidney Dis 2016; 67: 548–558 [DOI] [PubMed] [Google Scholar]
- 9. Tsubakihara Y, Nishi S, Akiba T. et al. 2008 Japanese Society for Dialysis Therapy: guidelines for renal anemia in chronic kidney disease. Ther Apher Dial 2010; 14: 240–275 [DOI] [PubMed] [Google Scholar]
- 10. Horl WH. Clinical aspects of iron use in the anemia of kidney disease. J Am Soc Nephrol 2007; 18: 382–393 [DOI] [PubMed] [Google Scholar]
- 11. Drueke TB, Locatelli F, Clyne N. et al. Normalization of hemoglobin level in patients with chronic kidney disease and anemia. N Engl J Med 2006; 355: 2071–2084 [DOI] [PubMed] [Google Scholar]
- 12. Pfeffer MA, Burdmann EA, Chen CY. et al. A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med 2009; 361: 2019–2032 [DOI] [PubMed] [Google Scholar]
- 13. Singh AK, Szczech L, Tang KL. et al. Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med 2006; 355: 2085–2098 [DOI] [PubMed] [Google Scholar]
- 14. Wish JB. Assessing iron status: beyond serum ferritin and transferrin saturation. Clin J Am Soc Nephrol 2006; 1(Suppl 1): S4–S8 [DOI] [PubMed] [Google Scholar]
- 15. Agarwal R, Rizkala AR, Bastani B. et al. A randomized controlled trial of oral versus intravenous iron in chronic kidney disease. Am J Nephrol 2006; 26: 445–454 [DOI] [PubMed] [Google Scholar]
- 16. Aggarwal HK, Nand N, Singh S. et al. Comparison of oral versus intravenous iron therapy in predialysis patients of chronic renal failure receiving recombinant human erythropoietin. J Assoc Physicians India 2003; 51: 170–174 [PubMed] [Google Scholar]
- 17. Charytan C, Qunibi W, Bailie GR.. Comparison of intravenous iron sucrose to oral iron in the treatment of anemic patients with chronic kidney disease not on dialysis. Nephron Clin Pract 2005; 100: c55–c62 [DOI] [PubMed] [Google Scholar]
- 18. Li H, Wang SX.. Intravenous iron sucrose in Chinese hemodialysis patients with renal anemia. Blood Purif 2008; 26: 151–156 [DOI] [PubMed] [Google Scholar]
- 19. Li H, Wang SX.. Intravenous iron sucrose in peritoneal dialysis patients with renal anemia. Perit Dial Int 2008; 28: 149–154 [PubMed] [Google Scholar]
- 20. Provenzano R, Schiller B, Rao M. et al. Ferumoxytol as an intravenous iron replacement therapy in hemodialysis patients. Clin J Am Soc Nephrol 2009; 4: 386–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Spinowitz BS, Kausz AT, Baptista J. et al. Ferumoxytol for treating iron deficiency anemia in CKD. J Am Soc Nephrol 2008; 19: 1599–1605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Van Wyck DB, Roppolo M, Martinez CO. et al. A randomized, controlled trial comparing IV iron sucrose to oral iron in anemic patients with nondialysis-dependent CKD. Kidney Int 2005; 68: 2846–2856 [DOI] [PubMed] [Google Scholar]
- 23. Fishbane S, Frei GL, Maesaka J.. Reduction in recombinant human erythropoietin doses by the use of chronic intravenous iron supplementation. Am J Kidney Dis 1995; 26: 41–46 [DOI] [PubMed] [Google Scholar]
- 24. Macdougall IC, Tucker B, Thompson J. et al. A randomized controlled study of iron supplementation in patients treated with erythropoietin. Kidney Int 1996; 50: 1694–1699 [DOI] [PubMed] [Google Scholar]
- 25. Nyvad O, Danielsen H, Madsen S.. Intravenous iron-sucrose complex to reduce epoetin demand in dialysis patients. Lancet 1994; 344: 1305–1306 [DOI] [PubMed] [Google Scholar]
- 26. Silverberg DS, Blum M, Agbaria Z. et al. The effect of i.v. iron alone or in combination with low-dose erythropoietin in the rapid correction of anemia of chronic renal failure in the predialysis period. Clin Nephrol 2001; 55: 212–219 [PubMed] [Google Scholar]
- 27. Macdougall IC, Geisser P.. Use of intravenous iron supplementation in chronic kidney disease: an update. Iran J Kidney Dis 2013; 7: 9–22 [PubMed] [Google Scholar]
- 28.AURYXIA prescribing information 2016. http://keryx.com/wp-content/uploads/Auryxia_PI_Keryx.pdf (27 June 2017, date last accessed)
- 29.Velphoro prescribing information 2014. http://velphoro.us/hcp (27 June 2017, date last accessed)
- 30. Fishbane S, Block GA, Loram L. et al. Effects of ferric citrate in patients with nondialysis-dependent CKD and iron deficiency anemia. J Am Soc Nephrol 2017; 28: 1851–1858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bailie GR, Clark JA, Lane CE. et al. Hypersensitivity reactions and deaths associated with intravenous iron preparations. Nephrol Dial Transplant 2005; 20: 1443–1449 [DOI] [PubMed] [Google Scholar]
- 32. Chertow GM, Mason PD, Vaage-Nilsen O. et al. On the relative safety of parenteral iron formulations. Nephrol Dial Transplant 2004; 19: 1571–1575 [DOI] [PubMed] [Google Scholar]
- 33. Chertow GM, Mason PD, Vaage-Nilsen O. et al. Update on adverse drug events associated with parenteral iron. Nephrol Dial Transplant 2006; 21: 378–382 [DOI] [PubMed] [Google Scholar]
- 34. European Medicines Agency. New recommendations to manage risk of allergic reactions with intravenous iron-containing medicines. 2013. http://www.ema.europa.eu/docs/en_GB/document_library/Referrals_document/IV_iron_31/WC500151308.pdf (27 June 2017, date last accessed)
- 35. Macdougall IC, Bircher AJ, Eckardt KU. et al. Iron management in chronic kidney disease: conclusions from a ‘Kidney Disease: Improving Global Outcomes’ (KDIGO) controversies conference. Kidney Int 2016; 89: 28–39 [DOI] [PubMed] [Google Scholar]
- 36. Drueke T, Witko-Sarsat V, Massy Z. et al. Iron therapy, advanced oxidation protein products, and carotid artery intima-media thickness in end-stage renal disease. Circulation 2002; 106: 2212–2217 [DOI] [PubMed] [Google Scholar]
- 37. Reis KA, Guz G, Ozdemir H. et al. Intravenous iron therapy as a possible risk factor for atherosclerosis in end-stage renal disease. Int Heart J 2005; 46: 255–264 [DOI] [PubMed] [Google Scholar]
- 38. Agarwal R, Vasavada N, Sachs NG. et al. Oxidative stress and renal injury with intravenous iron in patients with chronic kidney disease. Kidney Int 2004; 65: 2279–2289 [DOI] [PubMed] [Google Scholar]
- 39. Sunder-Plassmann G, Patruta SI, Horl WH.. Pathobiology of the role of iron in infection. Am J Kidney Dis. 1999; 34(Suppl 2): S25–S29 [DOI] [PubMed] [Google Scholar]
- 40. Weinberg ED. Iron availability and infection. Biochim Biophys Acta 2009; 1790: 600–605 [DOI] [PubMed] [Google Scholar]
- 41. Agarwal R, Kusek JW, Pappas MK.. A randomized trial of intravenous and oral iron in chronic kidney disease. Kidney Int 2015; 88: 905–914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ishida JH, Johansen KL.. Iron and infection in hemodialysis patients. Semin Dial 2014; 27: 26–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ishida JH, Marafino BJ, McCulloch CE. et al. Receipt of intravenous iron and clinical outcomes among hemodialysis patients hospitalized for infection. Clin J Am Soc Nephrol 2015; 10: 1799–1805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Winkelmayer WC, Goldstein BA, Mitani AA. et al. Safety of intravenous iron in hemodialysis: longer-term comparisons of iron sucrose versus sodium ferric gluconate complex. Am J Kidney Dis 2017; 69: 771–779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Canavese C, Bergamo D, Ciccone G. et al. Validation of serum ferritin values by magnetic susceptometry in predicting iron overload in dialysis patients. Kidney Int 2004; 65: 1091–1098 [DOI] [PubMed] [Google Scholar]
- 46. Ghoti H, Rachmilewitz EA, Simon-Lopez R. et al. Evidence for tissue iron overload in long-term hemodialysis patients and the impact of withdrawing parenteral iron. Eur J Haematol 2012; 89: 87–93 [DOI] [PubMed] [Google Scholar]
- 47. Rostoker G, Griuncelli M, Loridon C. et al. Hemodialysis-associated hemosiderosis in the era of erythropoiesis-stimulating agents: a MRI study. Am J Med 2012; 125: 991–999 [DOI] [PubMed] [Google Scholar]
- 48. Vaziri ND. Epidemic of iron overload in dialysis population caused by intravenous iron products: a plea for moderation. Am J Med 2012; 125: 951–952 [DOI] [PubMed] [Google Scholar]
- 49. Eschbach JW, Adamson JW.. Iron overload in renal failure patients: changes since the introduction of erythropoietin therapy. Kidney Int 1999; 55(Suppl 69): S35–S43 [DOI] [PubMed] [Google Scholar]
- 50. Avni T, Bieber A, Grossman A. et al. The safety of intravenous iron preparations: systematic review and meta-analysis. Mayo Clin Proc 2015; 90: 12–23 [DOI] [PubMed] [Google Scholar]
- 51. Rozen-Zvi B, Gafter-Gvili A, Paul M. et al. Intravenous versus oral iron supplementation for the treatment of anemia in CKD: systematic review and meta-analysis. Am J Kidney Dis 2008; 52: 897–906 [DOI] [PubMed] [Google Scholar]
- 52. Shepshelovich D, Rozen-Zvi B, Avni T. et al. Intravenous versus oral iron supplementation for the treatment of anemia in CKD: an updated systematic review and meta-analysis. Am J Kidney Dis 2016; 68: 677–690 [DOI] [PubMed] [Google Scholar]
- 53. Macdougall IC, Bock A, Carrera F. et al. The FIND-CKD study—a randomized controlled trial of intravenous iron versus oral iron in non-dialysis chronic kidney disease patients: background and rationale. Nephrol Dial Transplant 2014; 29: 843–850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Roger SD, Gaillard CA, Bock AH. et al. Safety of intravenous ferric carboxymaltose verus oral iron in patients with nondialysis-dependent CKD: and analysis of the 1-year FIND-CKD trial. Nephrol Dial Transplant 2017; 32: 1530–1539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Macdougall IC, Roger SD.. New data on the safety of IV iron-but why the discrepancy with FIND-CKD? Kidney Int 2015; 88: 1445–1446 [DOI] [PubMed] [Google Scholar]
- 56. Kshirsagar AV, Freburger JK, Ellis AR. et al. The comparative short-term effectiveness of iron dosing and formulations in US hemodialysis patients. Am J Med 2013; 126: 541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Brookhart MA, Freburger JK, Ellis AR. et al. Infection risk with bolus versus maintenance iron supplementation in hemodialysis patients. J Am Soc Nephrol 2013; 24: 1151–1158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Freburger JK, Ellis AR, Kshirsagar AV. et al. Comparative short-term safety of bolus versus maintenance iron dosing in hemodialysis patients: a replication study. BMC Nephrol 2014; 15: 154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Jahn MR, Andreasen HB, Futterer S. et al. A comparative study of the physicochemical properties of iron isomaltoside 1000 (Monofer), a new intravenous iron preparation and its clinical implications. Eur J Pharm Biopharm 2011; 78: 480–491 [DOI] [PubMed] [Google Scholar]
- 60. Neiser S, Rentsch D, Dippon U. et al. Physico-chemical properties of the new generation IV iron preparations ferumoxytol, iron isomaltoside 1000 and ferric carboxymaltose. Biometals 2015; 28: 615–635 [DOI] [PubMed] [Google Scholar]
- 61. Van Wyck D, Anderson J, Johnson K.. Labile iron in parenteral iron formulations: a quantitative and comparative study. Nephrol Dial Transplant 2004; 19: 561–565 [DOI] [PubMed] [Google Scholar]
- 62. Anirban G, Kohli HS, Jha V. et al. The comparative safety of various intravenous iron preparations in chronic kidney disease patients. Ren Fail 2008; 30: 629–638 [DOI] [PubMed] [Google Scholar]
- 63. Bhandari S, Kalra PA, Kothari J. et al. A randomized, open-label trial of iron isomaltoside 1000 (Monofer®) compared with iron sucrose (Venofer®) as maintenance therapy in haemodialysis patients. Nephrol Dial Transplant 2015; 30: 1577–1589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Macdougall IC, Strauss WE, McLaughlin J. et al. A randomized comparison of ferumoxytol and iron sucrose for treating iron deficiency anemia in patients with CKD. Clin J Am Soc Nephrol 2014; 9: 705–712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Charytan C, Bernardo MV, Koch TA. et al. Intravenous ferric carboxymaltose versus standard medical care in the treatment of iron deficiency anemia in patients with chronic kidney disease: a randomized, active-controlled, multi-center study. Nephrol Dial Transplant 2013; 28: 953–964 [DOI] [PubMed] [Google Scholar]
- 66. Bailie GR, Larkina M, Goodkin DA. et al. Variation in intravenous iron use internationally and over time: the Dialysis Outcomes and Practice Patterns Study (DOPPS). Nephrol Dial Transplant 2013; 28: 2570–2579 [DOI] [PubMed] [Google Scholar]