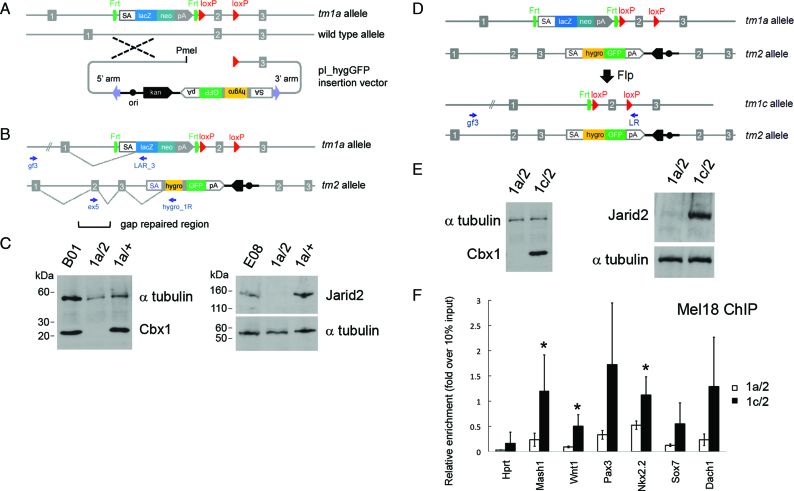
Figure 1.
Schematic of allele structures in second allele targeted and revertant ES cells and cell line validation. (A) Insertion-type targeting vector pI_hygGFP for inactivation of the WT allele in ES cells heterozygous for a standard IKMC knockout-first allele (tm1a). (B) Structure of the bi-allelic locus after targeting the second allele. The tm2 allele contains the pI_hygGFP targeting cassette and duplicated homology region, where exon 2 is re-generated by gap repair. (C) Western blots of Cbx1 and Jarid2 parental IKMC heterozygous ES cells (B01 and E08 lines), and examples of cell lines following pI_hygGFP electroporation including doubly targeted ES cells (1a/2) showing the absence of protein expression, and failed targeting events (1a/+). (D) Reversion from null mutant (1a/2) to conditional mutant (1c/2) by Flp recombinase. (E) Western blots of mutant (1a/2) and reverted (1c/2) Cbx1 and Jarid2 ES cell lines showing re-expression of protein. Primers for LR-PCR genotyping are indicated by small arrows and α-tubulin was used for Western blot loading controls. (F) Rescue of Polycomb PRC1 recruitment to PRC2 target genes in Jarid2 revertant cell lines (1c/2), shown by reinstatement of Mel18 binding at known Jarid2-dependent gene promoter regions, assessed by chromatin immunoprecipitation (ChIP)-qRT-PCR. Hprt is a control locus known to be negative for PRC1 binding. Results show mean ± s.d. of three biological replicates (independent cell lines), where values are expressed as relative fold-enrichment over 10% input chromatin. Asterisks indicate statistically significant differences between Jarid2 revertant (1c/2) and null (1a/2) cell lines (P < 0.05, one-tailed Student’s t-test).