Abstract
Background
Cardiovascular disease is the most common cause of mortality in haemodialysis (HD) patients and is highly predicted by markers of chronic inflammation. Regular exercise may have beneficial anti-inflammatory effects, but this is unclear in HD patients. This study assessed the effect of regular intradialytic exercise on soluble inflammatory factors and inflammatory leucocyte phenotypes.
Methods
Twenty-two HD patients from a centre where intradialytic cycling was offered thrice weekly and 16 HD patients receiving usual care volunteered. Exercising patients aimed to cycle for 30 min at rating of perceived exertion of ‘somewhat hard’. Baseline characteristics were compared with 16 healthy age-matched individuals. Physical function, soluble inflammatory markers and leucocyte phenotypes were assessed again after 6 months of regular exercise.
Results
Patients were less active than their healthy counterparts and had significant elevations in measures of inflammation [interleukin-6 (IL-6), C-reactive protein (CRP), tumour necrosis factor-α (TNF-α), intermediate and non-classical monocytes; all P < 0.001]. Six months of regular intradialytic exercise improved physical function (sit-to-stand 60). After 6 months, the proportion of intermediate monocytes in the exercising patients reduced compared with non-exercisers (7.58 ± 1.68% to 6.38 ± 1.81% versus 6.86 ± 1.45% to 7.88 ± 1.66%; P < 0.01). Numbers (but not proportion) of regulatory T cells decreased in the non-exercising patients only (P < 0.05). Training had no significant effect on circulating IL-6, CRP or TNF-α concentrations.
Conclusions
These findings suggest that regular intradialytic exercise is associated with an anti-inflammatory effect at a circulating cellular level but not in circulating cytokines. This may be protective against the increased risk of cardiovascular disease and mortality that is associated with chronic inflammation and elevated numbers of intermediate monocytes.
Keywords: cytokines, exercise, haemodialysis, inflammation, monocytes
Introduction
Haemodialysis (HD) patients have a vastly elevated risk of all-cause, and specifically cardiovascular, mortality [1], which is the most common cause of death in these patients. This increased risk of cardiovascular disease (CVD) morbidity and mortality is multifactorial but includes factors relating directly to kidney disease (e.g. the malnutrition–inflammation complex syndrome) and to the process of HD itself [2]. HD patients have chronically elevated circulating soluble markers of inflammation [including interleukin-6 (IL-6), C-reactive protein (CRP) and tumour necrosis factor-α (TNF-α)] that strongly associate with mortality and cardiovascular events [3]. Altered inflammatory immune cell populations and phenotypes are also evident in HD patients, including a shift towards the ‘pro-inflammatory’ CD16+ monocyte subpopulations [4, 5], which are also strongly associated with increased cardiovascular mortality in chronic kidney disease (CKD) [6].
Regular exercise is associated with reduced mortality and morbidity in various chronic diseases and is associated with a plethora of cardioprotective benefits [7]. The so-called ‘anti-inflammatory’ effect of regular exercise may play a key role in this through a number of different mechanisms. These include altering the balance of inflammatory immune cell phenotypes, reducing visceral adipose tissue mass and inflammatory cytokine release and, at higher intensities, through release of a potent anti-inflammatory cascade stimulated by muscle-derived cytokines [8, 9]. The importance of promoting an active lifestyle in the management of kidney disease is recognized in national and federal guidelines and in the scientific literature, as evidenced by a recent Cochrane Review [10], but the incorporation of exercise into clinical service has been slow and lags behind other long-term conditions. Within the HD population, patients are frequently inactive and the greater levels of inactivity are associated with an increased risk of mortality [11, 12]. However, despite reports in the literature of apparent anti-inflammatory effects of exercise in other patient populations [8, 9], including earlier stage of CKD [13], the impact of regular exercise in HD patients on inflammatory markers, such as CRP, IL-6 and TNF-α, remains unclear [14]. Furthermore, no studies in HD patients have determined the effect of regular exercise on inflammatory leucocyte phenotypes known to be central to chronic systemic inflammatory responses. This study aimed to determine the impact of a pragmatic 6-month intradialytic exercise programme on circulating soluble and cellular markers of chronic systemic inflammation.
Materials and methods
Participants
The study was approved by the East Midlands Research Ethics Committee (Northampton; 11/EM/0149). Additional ethical approval for the healthy cohort was granted by the Loughborough University Ethical Advisory Committee. All participants gave written informed consent.
Patients were recruited from two satellite HD units within the same renal network: one offered an intradialytic exercise programme as part of clinical care and one did not. An independent Consultant Nephrologist verified the medical suitability of all patients to exercise. Exclusion criteria for the study were: <18 years; established contraindications to exercise [15]; lower limb vascular access; recent clinically overt infection; already taking part in exercise programme; or an insufficient command of English to consent.
Following recruitment of the patient group, age-matched healthy participants were recruited from the local area. Healthy individuals completed a health screen and were ineligible if they had any history of kidney disease, diabetes, recent infection, CVD, smoking, or took any drugs known to affect digestion or metabolism. Healthy individuals donated a one-off serum blood sample and completed the same outcome measures as the HD patients (described below) within a laboratory setting.
Exercise programme
The exercise group participated in a progressive intradialytic exercise programme for 6 months; the non-exercising control group continued HD treatment as per their usual care. Exercise was provided in the form of a specially designed recumbent cycle (Letto series; Motomed, Reck, Germany). Patients aimed to cycle at a ‘somewhat hard’ rating of perceived exertion (RPE 12–14) [16] with supervised intradialytic exercise offered thrice weekly. The exercise programme was designed with a pragmatic approach; more details are provided in the Supplementary Material.
Randomization
An HD unit providing exercise and a unit not providing exercise were selected based on practicality and, therefore, the study was not randomized. All non-exercising patients were eligible to exercise if such a programme were available at their unit. This design reduced the sampling bias of using patients unable to, or not interested in, exercise as controls and ‘exercise-contamination’ of control patients that occurs when control and exercise patients share shifts.
Outcome measures
Blood samples were collected prior to HD at baseline and after 6 months (at least 48 h after previous exercise session); estimated glomerular filtration rate (eGFR) was calculated using the simplified Modification of Diet in Renal Disease equation [17]. Medical details were extracted from the patients’ clinical notes.
Plasma concentrations of CRP (IBL International GmbH, Hamburg, Germany), IL-6 and TNF-α (R&D systems, Abingdon, UK) were determined using commercially available high-sensitivity enzyme-linked immunosorbent assays. Assessment of monocyte phenotypes (classical: CD14++CD16−, intermediate: CD14++CD16+ and non-classical: CD14+CD16++) and regulatory T cells (Tregs: CD4+CD25+CD127low/−) via flow cytometry are described in the Supplementary Material.
Activity levels were determined using a tri-axial accelerometer (SenseWear, BodyMedia Inc., Pittsburgh, PA, USA). The participant wore the accelerometer for a single 7-day period collecting minute-by-minute data. Physical function was assessed using the sit-to-stand 60 (STS 60) test, which is validated against more detailed and lengthy assessment methods [18].
Omitted samples
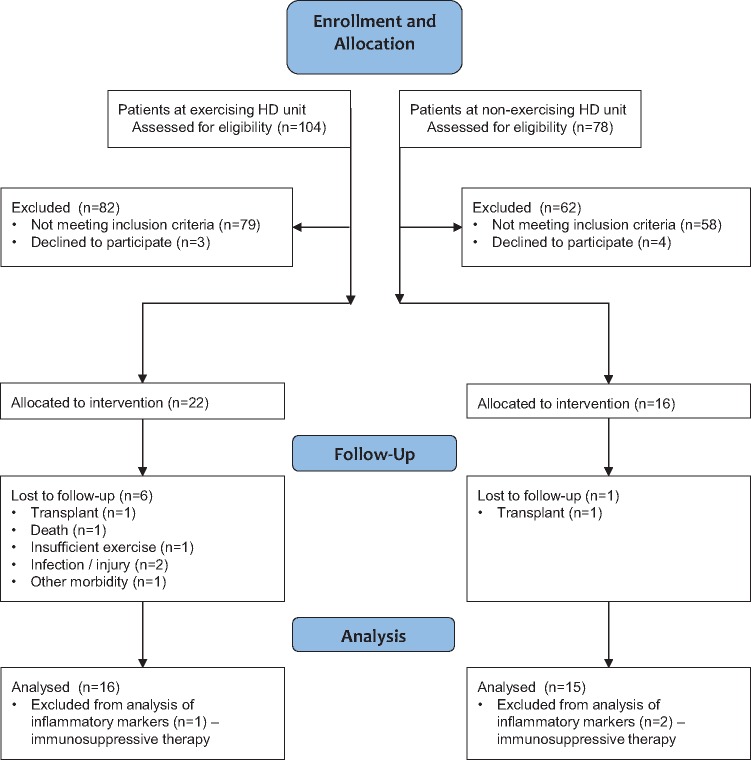
Patients on immunosuppressive therapy were excluded from analysis of inflammatory factors (Figure 1). Four exercise patients were omitted from analyses of monocyte phenotype due to unclear fluorescence patterns. Some patients declined physical function and activity outcomes; numbers for each outcome are presented in the Results.
Fig. 1.
Consolidated Standards of Reporting Trials diagram.
Sample size
Sample size for this study was pragmatic: all eligible patients in the two dialysis units were invited to participate (Figure 1).
Statistics
Data are presented as mean ± standard deviation or median (25th–75th percentiles) unless otherwise stated. Baseline comparisons between groups were completed using independent t-tests, non-parametric Mann–Whitney tests or chi-square tests where applicable. Two-factor mixed-measures analysis of variance (ANOVA) was used to analyse the effect of exercise: group (exercise versus control) × time (baseline versus 6 months). Where a significant group × time interaction was found, post hoc analysis explored the differences using paired t-tests within groups and independent t-tests for between-group differences in change from baseline at 6 months. Comparisons between treatment and non-treatment day activity levels were assessed using Wilcoxon signed-rank test. Effect sizes (ES) were calculated using Cohen’s D [19].
All statistical analyses were completed on Statistical Package for Social Sciences (SPSS v.21, IBM, New York, NY, USA). Statistical significance was accepted at P < 0.05.
Results
Recruitment and participant flow
A total of 38 HD patients participated in the study (Figure 1). Twenty-two patients were recruited into the exercise group, of whom 16 completed the 6-month study period (27.3% attrition rate). Sixteen non-exercising control HD patients volunteered, of whom 15 completed the study. Sixteen healthy participants volunteered and blood samples were obtained from 15.
Implementation of intervention
The exercising patients participated in 38 ± 12 exercise sessions. The mean session duration was 35 (30–42) min with patients exercising at 63 ± 8 r.p.m. and perceiving the exercise to be ‘somewhat hard’ [RPE: 12 (11–13)]. Mean power output was 16 ± 7 W.
Participant characteristics
The age and sex of the HD patients and healthy participants were similar (Table 1). The age of the exercising and non-exercising HD groups were different (57.0 ± 10.5 versus 70.2 ± 13.7 years; P = 0.005), with more South Asian patients in the exercise group (7/16 versus 1/15; P = 0.02), which reflected the demographic of the centres where recruitment took place. However, there were no differences in circulating or cellular inflammatory markers between the exercising and non-exercising groups.
Table 1.
Participant characteristicsa
| Healthy group (n = 16) | Combined HD patients (n = 31) | P-value | Exercising HD patients (n = 16) | Non-exercising HD patients (n = 15) | P-value | |
|---|---|---|---|---|---|---|
| Age (years) | 61.5 ± 10.9 | 63.4 ± 13.7 | 0.63 | 57.0 ± 10.5 | 70.2 ± 13.7 | 0.005* |
| Gender (male/female) | 8/8 | 18/13 | 0.37 | 8/8 | 10/5 | 0.35 |
| Weight (kg) | 75.3 ± 16.6 | 75.9 ± 23.1 | 0.92 | 71.0 ± 24.9 | 81.2 ± 20.6 | 0.23 |
| BMI (kg/m2) | 26.1 ± 5.0 | 27.1 ± 6.4 | 0.58 | 25.9 ± 6.7 | 28.4 ± 6.1 | 0.29 |
| Systolic blood pressure (mmHg) | 129 ± 13 | 136 ± 21 | 0.16 | 135 ± 24 | 138 ± 19 | 0.68 |
| eGFR (mL/min/1.73 m2) | 102 ± 22 | b | – | b | b | – |
| Number of comorbidities | – | – | – | 4 ± 2 | 4 ± 2 | 0.57 |
Data are presented as mean ± standard deviation.
All HD patients had established end-stage renal disease and had received HD treatment for at least 3 months.
Denotes a significant difference between exercising HD patient and non-exercising HD patient groups.
Outcomes
Inflammatory markers
HD patients had significantly higher concentrations of circulating IL-6 (ES = 1.32), TNF-α (ES = 1.37) and CRP (ES = 1.35) than the healthy participants (Table 2).
Table 2.
Comparisons in markers of inflammation and haematology at baseline between a healthy group and HD patients, and between the exercising and non-exercising HD groupsa
| Healthy group | HD group | P-value | Exercising HD group | Non-exercising HD group | P-value | |
|---|---|---|---|---|---|---|
| Markers of inflammation (n = 15 and n = 28) | (n = 15 and n = 13) | |||||
| IL-6 (pg/mL) | 0.81 (0.43–1.76) | 4.63 (2.73–7.09) | <0.001* | 5.39 ± 3.05 | 4.92 ± 2.79 | 0.68 |
| TNF-α (pg/mL) | 0.96 (0.60–1.73) | 3.21 (2.66–4.62) | <0.001* | 3.22 (2.72–4.24) | 3.20 (2.64–4.67) | 0.77 |
| CRP (mg/L) | 0.83 (0.21–1.90) | 4.61 (2.68–9.78) | <0.001* | 3.99 (2.34–6.86) | 7.89 (3.39–10.4) | 0.37 |
| Classical monocytes (%)b | 89.5 (87.0–92.1) | 80.3 (72.6–82.2) | <0.001* | 80.5 (75.4–82.3) | 79.8 (72.6–80.9) | 0.53 |
| Classical monocytes (cells/µL)b | 506 ± 130 | 516 ± 112 | 0.78 | 435 (410–525) | 580 (558–599) | 0.09 |
| Intermediate monocytes (%)b | 4.34 (3.74–4.95) | 6.77 (5.98–8.47) | <0.001* | 7.58 ± 1.68 | 6.86 ± 1.45 | 0.27 |
| Intermediate monocytes (cells/µL)b | 21.8 (19.4–29.1) | 45.9 (40.5–52.3) | <0.001* | 46.3 ± 14.7 | 49.4 ± 14.2 | 0.61 |
| Non-classical monocytes (%)b | 6.45 (4.2–8.5) | 13.6 (11.2–17.7) | <0.001* | 12.4 (10.8–16.4) | 13.8 (12.7–17.5) | 0.36 |
| Non-classical monocytes (cells/µL)b | 30.0 (23–50) | 90.9 (72.2–124.5) | <0.001* | 75.9 (59.9–105.7) | 96.9 (89.0–139.2) | 0.11 |
| Tregs (%)c | 7.67 ± 0.98 | 6.75 ± 1.53 | 0.02* | 6.93 ± 1.77 | 6.55 ± 1.24 | 0.53 |
| Tregs (cells/µL) | 20.1 (18.6–25.0) | 23.7 (17.3–36.0) | 0.53 | 23.6 (16.0–38.0) | 23.8 (19.1–32.4) | 0.57 |
| CD4+ lymphocytes (%)c | 20.2 (17.4–23.9) | 24.1 (20.1–28.9) | 0.18 | 23.9 ± 8.0 | 24.7 ± 7.2 | 0.79 |
| CD4+ lymphocytes (cells/µL) | 264 (227–369) | 366 (269–493) | 0.05* | 317 (253–496) | 369 (301–483) | 0.53 |
| Haematology (n = 15 and n = 31) | ||||||
| Haemoglobin (g/L) | 134 ± 17 | 118 ± 12 | 0.003* | 126 (118–131) | 105 (102–110) | <0.001† |
| Red blood cells (×1012/L) | 4.49 ± 0.44 | 3.67 ± 0.48 | <0.001* | 3.81 ± 0.58 | 3.55 | 0.14 |
| White blood cells (×109/L) | 5.14 ± 0.95 | 7.05 ± 2.56 | 0.001* | 6.64 ± 2.44 | 7.47 | 0.38 |
| Neutrophils (×109/L) | 2.8 (2.4–3.5) | 3.9 (3.0–5.6) | 0.005* | 3.6 (2.9–5.6) | 4.3 (3.7–5.9) | 0.32 |
| Monocytes (×109/L) | 0.5 (0.5–0.7) | 0.7 (0.5–0.8) | 0.24 | 0.6 (0.5–0.7) | 0.7 (0.6–0.8) | 0.054 |
| Lymphocytes (×109/L) | 1.5 ± 0.4 | 1.6 ± 0.6 | 0.45 | 1.58 ± 0.66 | 1.68 | 0.67 |
Data are presented as mean ± standard deviation or median (25th–75th percentiles). P-values are derived from independent t-tests or Mann–Whitney test, where applicable. Classical monocytes refer to CD14++CD16−; intermediate monocytes to CD14++CD16+; non-classical monocytes to CD14+CD16++. Tregs (%) and (cells/µL) refer to the percentage and number of CD4+ lymphocytes that are CD25+CD127low/−.
For monocytes n = 24 for HD group; n = 11 for exercise HD group and n = 13 for non-exercising HD group.
For percent Tregs and percent CD4+ lymphocytes n = 16 for healthy group.
Denotes a significant difference between healthy and HD groups.
Denotes a significant difference between exercising and non-exercise groups.
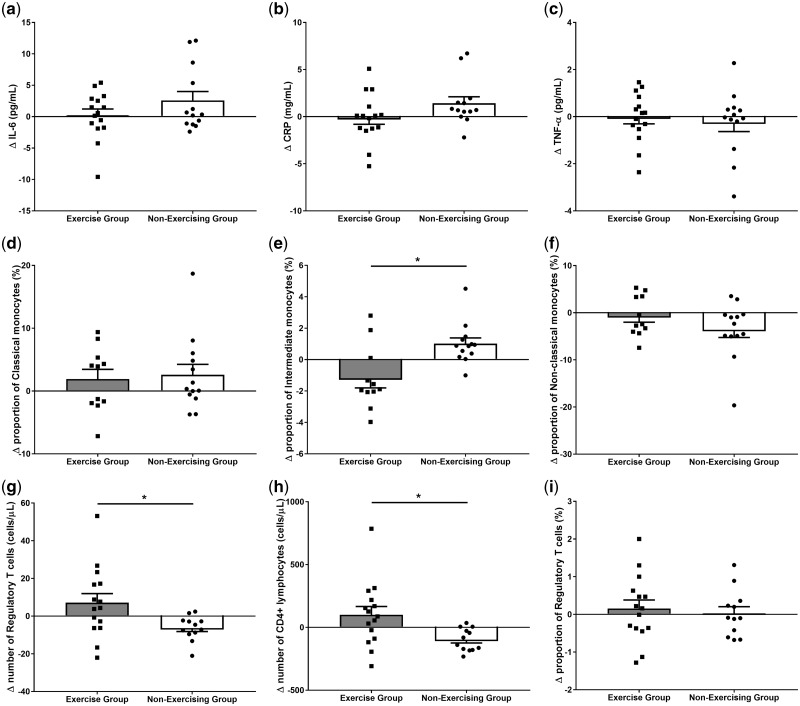
Circulating concentrations of IL-6, CRP and TNF-α did not differ between the exercising and non-exercising patients at baseline (Table 2). ANOVA found no interaction or main effects in response to the 6-month exercise programme (Figure 2a–c).
Fig. 2.
Changes in indices of systemic inflammation after 6 months in the exercise and non-exercise HD patients. (a) IL-6; (b) CRP; (c) TNF-α; the proportion of monocytes that are (d) classical, (e) intermediate, (f) non-classical phenotype; (g) number of Tregs; (h) number of CD4+ lymphocytes; (i) proportion of CD4+ lymphocytes that are Tregs. Each black square and circle represent an exercising and non-exercising patient, respectively; bars show mean ± standard error of the mean. Asterisks denotes a significant difference in change between exercising and non-exercising patients (P < 0.05). Exercising patients: n = 15 (except for monocytes where n = 11), non-exercising patients: n = 13.
Monocytes
The proportion and number of intermediate (ES = 1.23 and ES = 1.17, respectively) and non-classical monocytes (ES = 1.56 and ES = 1.31) were significantly greater in HD patients compared with healthy participants (Table 2).
There were no significant baseline differences between the exercising and non-exercising HD patients in the proportion of classical, intermediate or non-classical monocytes (Table 2). ANOVA revealed a significant group × time interaction in the proportion (F = 10.4, P = 0.004) and number (F = 4.91, P = 0.04) of intermediate monocytes between the exercising and non-exercising HD patients, whereas classical and non-classical monocytes did not show any significant effects (Figure 2d–f). Post hoc tests showed a trend for reduced proportion of intermediate monocytes in the exercising patients (7.58 ± 1.68% to 6.38 ± 1.81%; P = 0.08, ES = 0.68), and an increase in non-exercising HD patients (6.86 ± 1.45% to 7.88 ± 1.66%; P = 0.02, ES = 0.65). The magnitude of change in the proportion of intermediate monocytes observed in the exercising patients was significantly different from the non-exercising patients (P = 0.004).
Tregs
The number of circulating CD4+ lymphocytes were higher in the HD patients compared with the healthy participants (ES = 0.71, Table 2). However, the proportion of circulating CD4+ lymphocytes that were Tregs was lower in the HD patients (ES = 0.72, Table 2). The number of Tregs did not differ between groups.
At baseline, there were no significant differences between the exercising and non-exercising HD patients in the proportion or number of Tregs or CD4+ lymphocytes (Table 2).
ANOVA revealed group × time interactions in the proportion and number of CD4+ lymphocytes (F = 11.3, P = 0.002 and F = 12.7, P = 0.001, Figure 2h). In both cases, post hoc paired t-tests revealed a significant decrease in the non-exercising patients from baseline to 6 months (P < 0.001, ES = 0.67 and P = 0.001, ES = 0.61, respectively) with no significant change in the exercise group.
ANOVA also revealed a group × time interaction in the number of Tregs (F = 9.89, P = 0.004). Post hoc paired t-tests revealed a trend for an increase in exercising patients (P = 0.10, ES = 0.23) and a decrease in non-exercising patients (P = 0.003, ES = 0.50; Figure 2g). The magnitude of change in the number of Tregs observed over the 6 months in the exercising patients was significantly different from that seen in the non-exercising patients (P = 0.02). There was no change in the proportion of CD4+ lymphocytes that were Tregs (interaction: P = 0.74, Figure 2i).
Physical function
Compared with healthy participants, HD patients had significantly lower levels of physical function, as measured by the STS 60 test (ES = 1.28, Table 3).
Table 3.
Comparisons in physical activity and function between a healthy group and HD patients, and between the exercising and non-exercising HD groupsa
| Healthy group | HD group | P-value | Exercising HD group | Non-exercising HD group | P-value | |
|---|---|---|---|---|---|---|
| Physical activity and function | (n = 16) | (n = 24) | (n = 10) | (n = 14) | ||
| Energy expenditure (kJ/day) | 10505 ± 1495 | 9446 ± 2368 | 0.12 | 9315 ± 2703 | 9540 ± 2200 | 0.82 |
| Steps (per day) | 7594 (5847–9539) | 2934 (2058–4460) | <0.001* | 2237 (1995–3902) | 3234 (2527–4462) | 0.47 |
| Time being physically active (min/day) | 105 (86–142) | 55 (26–98) | 0.009* | 63 (49–102) | 51 (16–87) | 0.26 |
| Time being sedentary (min/day) | 1335 (1295–1354) | 1385 (1342–1413) | 0.009* | 1389 (1353–1424) | 1376 (1338–1390) | 0.26 |
| STS 60 (reps)b | 28 ± 13 | 13 ± 9 | 0.001* | 13 ± 10 | 13 ± 9 | 0.84 |
Data are presented as mean ± standard deviation or median (25th–75th percentiles). P-values are derived from independent t-tests or Mann–Whitney test, where applicable.
For STS 60: n = 23 for HD group, n = 11 for exercise HD group and n = 12 for non-exercising HD group.
Denotes a significant difference between healthy and HD groups.
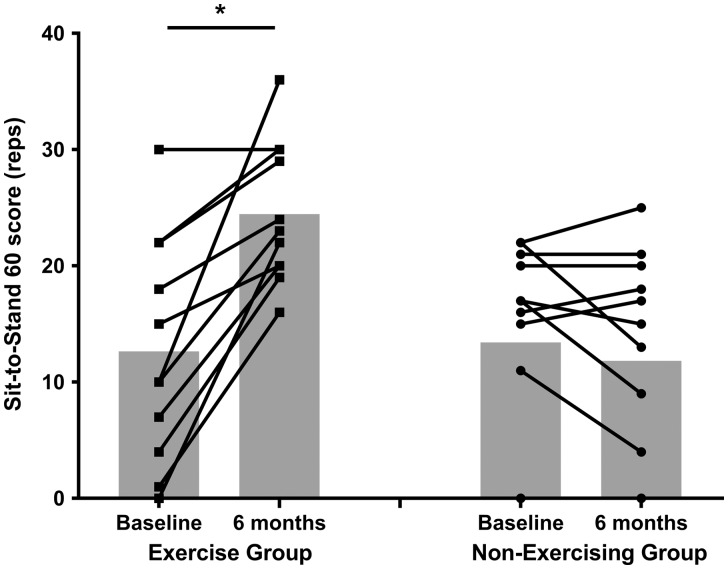
At baseline, there were no significant differences between the exercising and non-exercising HD groups (P = 0.88). Change in physical function was different between groups (F = 28.1, P < 0.001, Figure 3), with increases in the exercise group (P < 0.001, ES = 1.47), but not the non-exercising group (P = 0.21, ES = 0.18).
Fig. 3.
STS 60 score at baseline and 6 months in exercising and non-exercising HD patients. Lines represent individual patients; grey bars show the mean. Asterisk denotes a significant change from baseline (P < 0.001); exercise group: n = 11, non-exercising group: n = 12.
Activity levels
Twenty-four HD patients and 16 healthy participants provided usable accelerometer data. Habitual activity levels were lower in the HD patients than the healthy cohort (Table 3). The number of steps completed each day (ES = 1.14) and the time spent being physically active were greater in the healthy group (ES = 0.50). There were no differences between HD subgroups (Table 3).
Patients’ activity levels were significantly reduced on HD treatment days compared with non-treatment days, including number of steps [2100 (1643–3015) versus 3279 (2350–5055) steps/day; P < 0.001, ES = 0.38] and non-sedentary time [52.4 (15.7–95.5) versus 57.2 (27.5–106.0) min/day; P = 0.04, ES = 0.05].
Anthropometric and clinical parameters
Regular exercise did not induce changes in weight, body mass index (BMI), resting blood pressures, medications or haematology (all P > 0.05).
Discussion
This study is the first to demonstrate that completion of a 6-month intradialytic exercise programme promotes a shift away from the pro-inflammatory and pro-atherogenic intermediate monocyte phenotype towards an anti-inflammatory circulating leucocyte profile, as evidenced by an increase in the number of the anti-inflammatory Treg phenotype, compared with non-exercising patients. We also confirm elevated circulating and cellular markers of systemic inflammation in HD patients compared with an age-matched healthy cohort: CRP, IL-6 and TNF-α were significantly elevated in the HD cohort, the proportion of Tregs was reduced and monocyte populations shifted towards intermediate and non-classical phenotypes. Physical activity levels and physical function were also lower in HD patients.
This is the first study in any clinical population to show that exercise training selectively diminishes the proportion of monocytes within the inflammatory intermediate subset. Elsewhere, in an inactive elderly, but healthy group, 12 weeks of thrice weekly endurance and resistance exercise training significantly reduced the percentage of CD14+CD16+ monocytes and decreased the TNF-α response to lipopolysaccharide stimulation [20]. However, CD16+ monocytes were not analysed separately as intermediate and non-classical subsets, as this is a relatively new classification system [21]. Whether the present findings are applicable to populations other than HD patients is not known, but may explain these previous observed changes in ‘CD16+’ monocytes.
Elevations in circulating intermediate monocytes are associated with an increased cardiovascular risk in non-CKD, CKD and HD patients [4, 6, 22] and have been described as a promising therapeutic target for CVD in CKD [23]. In agreement with the present study, the number and proportion of intermediate monocytes are increased in HD patients compared with healthy cohorts [4, 5]. Intermediate monocytes secrete pro-inflammatory cytokines (TNF-α, IL-1β and IL-6), express toll-like receptor 4 and adhesion molecules (CCR5 and CX3CR1) and exhibit spontaneous reactive oxygen species production [24–26], all considered mechanisms for cardiovascular dysfunction. The reduction in intermediate monocytes that we report here is encouraging as it may be a mechanism by which exercise protects against systemic inflammation and associated cardiovascular disorders in HD patients.
In the present study, the proportion of CD4+ lymphocytes that were Tregs was lower in HD patients than in the healthy group. Reduced Treg capacity has been reported previously in HD patients [27, 28]. Abnormalities in Treg activity are harmful; deficiencies are associated with autoimmunity, inflammation and allergy, whereas over-activation is associated with increased risk of chronic infections and tumour growth [29]. The diminished Treg capacity in HD patients may contribute to chronic immune activation and inflammation typically exhibited in this population.
The favourable change in the number of Tregs in the exercise group after 6 months compared with the non-exercising group may represent enhanced anti-inflammatory capacity. Sedentary but otherwise healthy individuals have demonstrated lower numbers of Tregs than physically active groups and antigen-stimulated IL-10 release correlates with this cell population [30]. However, as we found no change in the proportion of CD4+ lymphocytes that were Tregs, the increase we observed may simply relate to differences in overall CD4+ numbers. This is further supported by the observation that the number of CD4+ lymphocytes decreased significantly in the non-exercising group but not in the exercise group. Therefore, it is not clear whether the enhanced Treg population in the exercise group actually represents a true anti-inflammatory adaptation.
Circulating CRP, IL-6 and TNF-α were not improved after 6 months of regular intradialytic exercise in this study. Examination of the literature to date reveals that no well-controlled trials have reported significant meaningful reductions in these soluble inflammatory markers after exercise training in HD patients [14]. Well-designed studies have reported no change in CRP after 3–4 months of intradialytic cycling or extradialytic resistance training [31, 32], or with maintained CRP but no improvement in IL-6 or TNF-α [33, 34]. In pre-dialysis CKD cohorts, resistance exercise that increases muscle mass [35] and regular walking exercise have been shown to favourably alter circulating inflammatory factors [13].
In a large cohort of healthy individuals (n = 4289), physical activity was associated with reduced CRP and IL-6, and increasing activity levels reduced markers of inflammation over a 10-year follow-up [36]. Therefore, for these soluble inflammatory markers, a large cohort or a long exercise intervention may be required to detect meaningful change. This is likely exacerbated in HD patients by the large intra- and inter-individual variation of IL-6, CRP and TNF-α. The day-to-day variation (i.e. due to fluid overload, recurrent infections, HD itself) in this population may limit the use of CRP and circulating cytokines in isolation to detect long-term change.
Previous studies have demonstrated sedentary behaviour in HD patients [11, 37], but the extent of inactivity is worth highlighting. Significantly, the least active HD patients have a 62% increased mortality risk [12] and those with the lowest physical function have higher hospitalization rates and all-cause mortality [38, 39]. A recent Cochrane Review concludes there are various benefits of increasing physical activity for health and well-being in CKD [10], and the present study adds to this body of evidence. Despite the seemingly low power output the exercise group improved physical function. This improvement was probably augmented by low baseline values giving greater potential for improvement. Increasing physical function has a positive impact on quality of life and is valued very highly by patients; therefore, promotion of this key outcome is likely to be more effective in encouraging continued participation in physical activity.
This exercise programme was limited to one mode and prescription of exercise and was not standardized per patient; different exercise modalities, at different times or intensities, may have different results. Nonetheless, this study makes a major contribution because it was pragmatic in design, thereby ascertaining a realistic response to an achievable intradialytic programme per se in a naturally heterogeneous HD cohort. There was, therefore, expected variation in the absolute exercise intensity achieved in this study (comparable to other studies [31, 40]); however, subjective intensity remained quite consistent, as evidenced by RPE.
For reasons of practicality and to avoid sampling bias and ‘exercise-contamination’ of control patients that occurs when control and exercise patients share shifts, this study was not randomized. This led to different demographics between the exercising and non-exercising groups, including ethnicity and age, but did not confound the key outcome measures. Likewise, haemoglobin was different between groups at baseline, but as this did not change over time, it is unlikely to have influenced the findings. Finally, the small sample size is due to the practicalities of running such a study despite approaching all eligible patients. This pragmatic evaluation of intradialytic exercise provides important novel evidence into anti-inflammatory adaptations that provides a primary point of reference for future investigations into the therapeutic benefits of exercise in this population.
Conclusions
HD patients are sedentary and demonstrate high levels of chronic inflammation, yet we show for the first time that undertaking regular exercise during HD has an anti-inflammatory effect at a circulating cellular level. The reduction in intermediate monocytes may be protective against the substantially increased risk of cardiovascular morbidity and mortality and further supports the therapeutic potential of regular exercise in these patients.
Supplementary data
Supplementary data are available online at http://ckj.oxfordjournals.org.
Supplementary Material
Acknowledgements
We thank all the staff and patients who gave their time to participate in the study. Dr James Medcalf and Dr Richard Baines assessed patient eligibility. The results of this study have been reported honestly, accurately and without fabrication, falsification or inappropriate data manipulation.
Funding
The work was generously funded by the Stoneygate Trust and the Leicester Kidney Care Appeal, and supported by the National Institute for Health Research (NIHR) Diet, Lifestyle & Physical Activity Biomedical Research Unit based at University Hospitals of Leicester and Loughborough University. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.
Authors’ contributions
Research idea and study design: N.C.B. and A.C.S.; data acquisition: M.D., H.M.L.Y and D.R.C.; data analysis/interpretation: M.D.; and supervision or mentorship: N.C.B., A.C.S. and J.O.B. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Conflict of interest statement
Outside the submitted work, Reck UK (manufacturers of the exercise bike) funded M.D., H.M.L.Y. and J.O.B. to attend the 2012 BMJ Awards. There are no other financial conflicts of interest.
References
- 1. Sarnak MJ, Levey AS, Schoolwerth AC. et al. Kidney disease as a risk factor for development of cardiovascular disease a statement from the American Heart Association Councils on kidney in cardiovascular disease, high blood pressure research, clinical cardiology, and epidemiology and prevention. Circulation 2003; 108: 2154–2169 [DOI] [PubMed] [Google Scholar]
- 2. Kalantar-Zadeh K, Ikizler TA, Block G. et al. Malnutrition-inflammation complex syndrome in dialysis patients: causes and consequences. Am J Kidney Dis 2003; 42: 864–881 [DOI] [PubMed] [Google Scholar]
- 3. Stenvinkel P. Malnutrition and chronic inflammation as risk factors for cardiovascular disease in chronic renal failure. Blood Purif 2001; 19: 143–151 [DOI] [PubMed] [Google Scholar]
- 4. Heine GH, Ulrich C, Seibert E. et al. CD14 ++CD16+ monocytes but not total monocyte numbers predict cardiovascular events in dialysis patients. Kidney Int 2008;73: 622–629 [DOI] [PubMed] [Google Scholar]
- 5. Merino A, Buendia P, Martin-Malo A. et al. Senescent CD14+ CD16+ monocytes exhibit proinflammatory and proatherosclerotic activity. J Immunol 2011; 186: 1809–1815. [DOI] [PubMed] [Google Scholar]
- 6. Rogacev KS, Seiler S, Zawada AM. et al. CD14 ++ CD16+ monocytes and cardiovascular outcome in patients with chronic kidney disease. Eur Heart J 2011; 32: 84–92 [DOI] [PubMed] [Google Scholar]
- 7. Pedersen BK, Saltin B.. Evidence for prescribing exercise as therapy in chronic disease. Scand J Med Sci Sports 2006; 16: 3–63 [DOI] [PubMed] [Google Scholar]
- 8. Beavers KM, Brinkley TE, Nicklas BJ.. Effect of exercise training on chronic inflammation. Clin Chim Acta 2010; 411: 785–793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gleeson M, Bishop NC, Stensel DJ. et al. The anti-inflammatory effects of exercise: mechanisms and implications for the prevention and treatment of disease. Nat Rev Immunol 2011; 11: 607–615 [DOI] [PubMed] [Google Scholar]
- 10. Heiwe S, Jacobson SH.. Exercise training for adults with chronic kidney disease. Cochrane Database Syst Rev 2011; 10: 1–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Johansen KL, Chertow GM, Ng AV. et al. Physical activity levels in patients on hemodialysis and healthy sedentary controls. Kidney Int 2000; 57: 2564–2570 [DOI] [PubMed] [Google Scholar]
- 12. O'Hare AM, Tawney K, Bacchetti P. et al. Decreased survival among sedentary patients undergoing dialysis: results from the dialysis morbidity and mortality study wave 2. Am J Kidney Dis 2003; 41: 447–454 [DOI] [PubMed] [Google Scholar]
- 13. Viana JL, Kosmadakis GC, Watson EL. et al. Evidence for anti-inflammatory effects of exercise in CKD. J Am Soc Nephrol 2014; 25: 2121–2130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dungey M, Hull KL, Smith AC. et al. Inflammatory factors and exercise in chronic kidney disease. Int J Endocrinol 2013; 2013: 569831 [published online] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. American College of Sports Medicine. ACSM's Guidelines for Exercise Testing and Prescription .9th edn Philadelphia, PA: Lippincott Williams & Wilkins, 2013 [Google Scholar]
- 16. Borg GAV. Perceived exertion: a note on ‘history’ and methods. Med Sci Sport Exer 1973; 5: 90–93 [PubMed] [Google Scholar]
- 17. Levey AS, Greene T, Kusek JW. et al. A simplified equation to predict glomerular filtration rate from serum creatinine. J Am Soc Nephrol 2000; 11(S2): 155 [Google Scholar]
- 18. McIntyre CW, Selby NM, Sigrist M. et al. Patients receiving maintenance dialysis have more severe functionally significant skeletal muscle wasting than patients with dialysis-independent chronic kidney disease. Nephrol Dial Transplant 2006; 21: 2210–2216 [DOI] [PubMed] [Google Scholar]
- 19. Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd edn Hillsdale, NJ: Lawrence Erlbaum Associates, 1988 [Google Scholar]
- 20. Timmerman KL, Flynn MG, Coen PM. et al. Exercise training-induced lowering of inflammatory (CD14+ CD16+) monocytes: a role in the anti-inflammatory influence of exercise? J Leukocyte Biol 2008; 84: 1271–1278 [DOI] [PubMed] [Google Scholar]
- 21. Ziegler-Heitbrock L, Hofer TP.. Toward a refined definition of monocyte subsets. Front Immunol 2013; 4: 1–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rogacev KS, Cremers B, Zawada AM. et al. CD14 ++ CD16+ monocytes independently predict cardiovascular events: a cohort study of 951 patients referred for elective coronary angiography. J Am Coll Cardiol 2012; 60: 1512–1520 [DOI] [PubMed] [Google Scholar]
- 23. Heine GH, Ortiz A, Massy ZA. et al. Monocyte subpopulations and cardiovascular risk in chronic kidney disease. Nat Rev Nephrol 2012; 8: 362–369 [DOI] [PubMed] [Google Scholar]
- 24. Cros J, Cagnard N, Woollard K. et al. Human CD14dim monocytes patrol and sense nucleic acids and viruses via TLR7 and TLR8 receptors. Immunity 2010; 33: 375–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rossol M, Kraus S, Pierer M. et al. The CD14brightCD16+ monocyte subset is expanded in rheumatoid arthritis and promotes expansion of the Th17 cell population. Arthritis Rheum 2012; 64: 671–677 [DOI] [PubMed] [Google Scholar]
- 26. Shantsila E, Wrigley B, Tapp L. et al. Immunophenotypic characterization of human monocyte subsets: possible implications for cardiovascular disease pathophysiology. J Thromb Haemost 2011; 9: 1056–1066 [DOI] [PubMed] [Google Scholar]
- 27. Hendrikx TK, van Gurp EA, Mol WM. et al. End-stage renal failure and regulatory activities of CD4+CD25bright+FoxP3+ T-cells. Nephrol Dial Transplant 2009; 24: 1969–1978 [DOI] [PubMed] [Google Scholar]
- 28. Topal C, Köksoy S, Süleymanlar G. et al. Peripheral Treg count and it’s determinants in unsensitized and sensitized chronic kidney disease patients. Int Urol Nephrol 2013; 45: 1647–1652 [DOI] [PubMed] [Google Scholar]
- 29. Shalev I, Schmelzle M, Robson SC. et al. Making sense of regulatory T cell suppressive function. Semin Immunol 2011; 23: 282–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30). Handzlik MK, Shaw AJ, Dungey M. et al. The influence of exercise training status on antigen-stimulated IL-10 production in whole blood culture and numbers of circulating regulatory T cells. Eur J Appl Physiol 2013; 113: 1839–1848 [DOI] [PubMed] [Google Scholar]
- 31. Kopple JD, Wang H, Casaburi R. et al. Exercise in maintenance hemodialysis patients induces transcriptional changes in genes favoring anabolic muscle. J Am Soc Nephrol 2007; 18: 2975–2986 [DOI] [PubMed] [Google Scholar]
- 32. Wilund KR, Tomayko EJ, Wu PT. et al. Intradialytic exercise training reduces oxidative stress and epicardial fat: a pilot study. Nephrol Dial Transplant 2010; 25: 2695–2701 [DOI] [PubMed] [Google Scholar]
- 33. Cheema BSB, Abas H, Smith BC. et al. Effect of resistance training during hemodialysis on circulating cytokines: a randomized controlled trial. Eur J Appl Physiol 2011; 111: 1437–1445 [DOI] [PubMed] [Google Scholar]
- 34. Cheema BSB, Abas H, Smith BC. et al. Progressive exercise for anabolism in kidney disease (PEAK): a randomized, controlled trial of resistance training during hemodialysis. J Am Soc Nephrol 2007; 18: 1594–1601 [DOI] [PubMed] [Google Scholar]
- 35. Castaneda C, Gordon PL, Parker RC. et al. Resistance training to reduce the malnutrition-inflammation complex syndrome of chronic kidney disease. Am J Kidney Dis 2004; 43: 607–616 [DOI] [PubMed] [Google Scholar]
- 36. Hamer M, Sabia S, Batty GD. et al. Follow-up in men and women from the Whitehall II cohort study. Circulation 2012; 126: 928–933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Baria F, Kamimura MA, Avesani CM. et al. Activity-related energy expenditure of patients undergoing hemodialysis. J Renal Nutr 2010; 21: 226–234 [DOI] [PubMed] [Google Scholar]
- 38. Mapes DL, Lopes AA, Satayathum S. et al. Health-related quality of life as a predictor of mortality and hospitalization: the Dialysis Outcomes and Practice Patterns Study (DOPPS). Kidney Int 2003; 64: 339–349 [DOI] [PubMed] [Google Scholar]
- 39. Vogt BP, Borges MC, Goés CR. et al. Handgrip strength is an independent predictor of all-cause mortality in maintenance dialysis patients. Clin Nutr 2016; 35: 1429–1433 [DOI] [PubMed] [Google Scholar]
- 40. Storer TW, Casaburi R, Sawelson S. et al. Endurance exercise training during haemodialysis improves strength, power, fatigability and physical performance in maintenance haemodialysis patients. Nephrol Dial Transplant 2005; 20: 1429–1437 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.