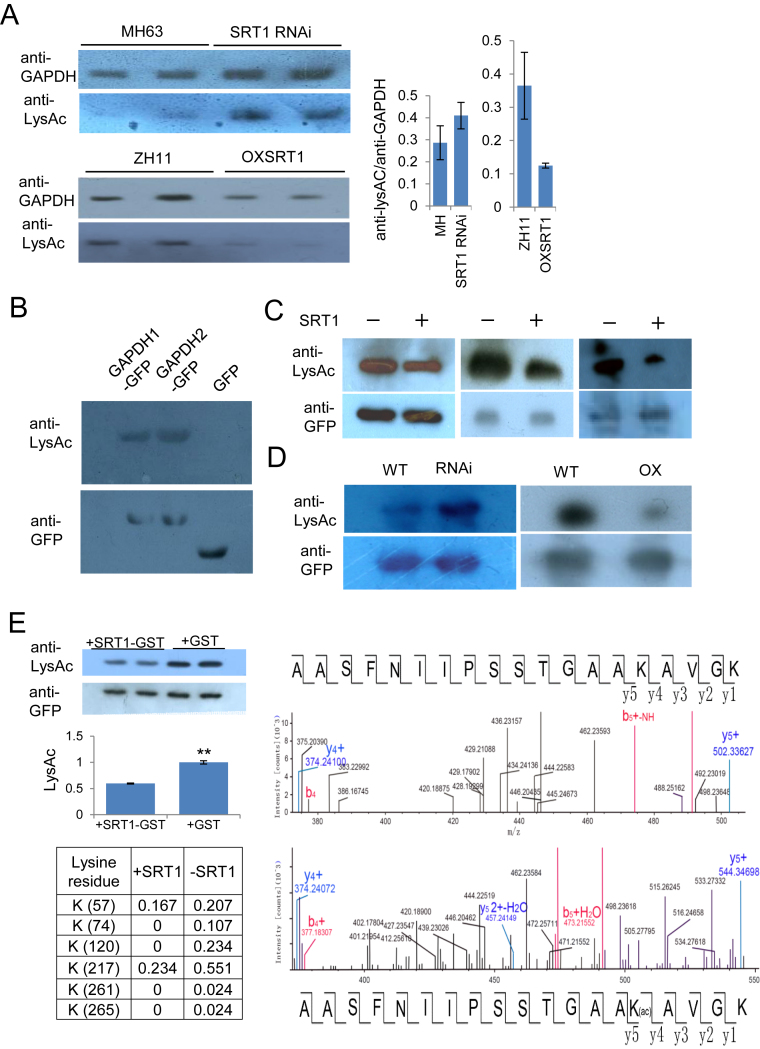
Figure 5.
OsSRT1 deacetylates OsGAPDH1. (A) Lysine acetylation of rice endogenous GAPDH is regulated by OsSRT1. Nuclear proteins isolated from wild type (MH63 and ZH11), OsSRT1 RNAi and OXSRT1 transgenic plants leaves were immunoprecipitated by anti-GAPDH and analyzed by immunoblots using anti-GFP and anti-LysAc (acetylated lysine residues). Relative LysAc/GAPDH signals in the different genotypes are measured by ImageJ (right). Bars are means ± SD from three measures of each of the two repeats. (B) In vitro acetylation of OsGAPDH1 and OsGAPDH2. Transiently expressed OsGAPDH1-GFP, OsGAPDH2-GFP or GFP alone were purified from tobacco cell extracts with anti-GFP beads, analyzed by immunoblots with anti-LysAc and anti-GFP as controls. (C) OsSRT1 reduces OsGAPDH1 acetylation levels. Tobacco leaves cells transiently expressed with 35S:GAPDH1-GFP together with (+) or without (–) 35S:OsSRT1-FLAG, immunoprecipitated with anti-GFP, and analyzed by immunoblots with anti-LysAc and anti-GFP. (D) OsSRT1 reduces OsGAPDH1 acetylation levels in rice cells. Protoplasts of wild type (WT), OsSRT1 RNAi or over-expression (OXSRT1) leaves were transiently transfected with 35S:OsGAPDH1-GFP. Nuclear proteins of transfected protoplast were immunoprecipitated by anti-GFP and analyzed by immunoblots with anti-GFP and anti-LysAc, respectively and indicated at left. (E) Left upper part, OsSRT1 deacetylates OsGAPDH1 in vitro. Escherichia coli-produced OsSRT1-GST protein was incubated with tobacco cell-produced OsGAPDH1-GFP. Anti-GFP was used for loading controls and anti-LysAc detected lysine acetylation levels of OsGAPDH1-GFP in the absence or presence of OsSRT1-GST. Left lower part, acetylation ratios of indicated lysine residues of OsGAPDH1 detected by IP-MS analysis of tobacco leaf cell-expressed GAPDH1-GFP protein (0.5 μg) in the presence or absence of OsSRT1-GST (3 μg). Right, molecular mass of an OsGAPDH1 peptide without (upper) or with (lower) K217 acetylation (Ac) detected by MS.