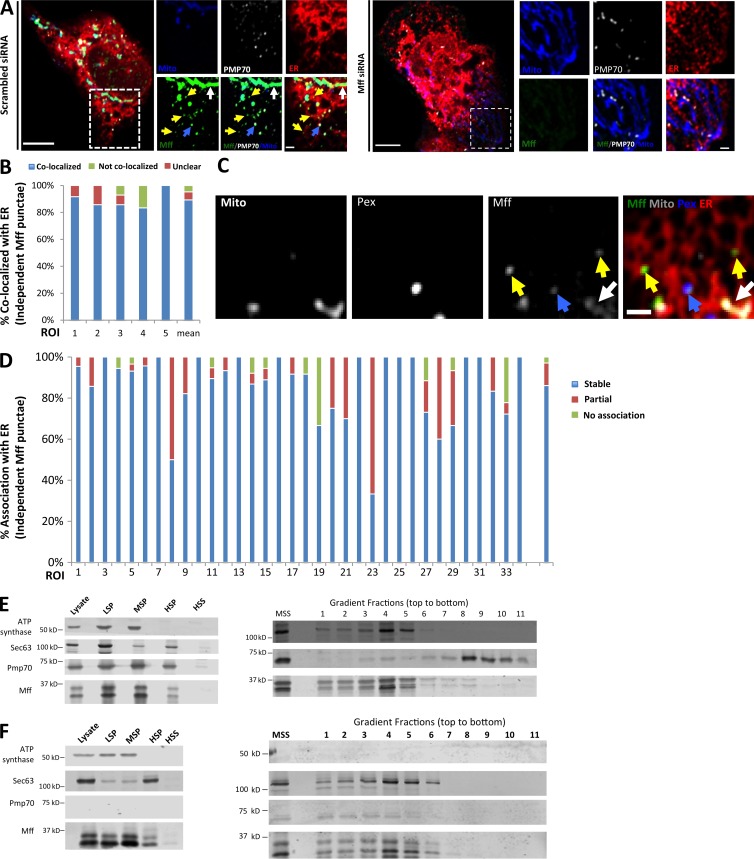
Figure 4.
A subpopulation of Mff localizes to ER. (A) Endogenous Mff localization in a fixed U2OS cell by immunofluorescence. Cells labeled with anti-Tom20 (mitochondria, blue), anti-PMP70 (peroxisomes, gray), anti-Mff (green) and transfected with ER-TagRFP (ER, red). Left, scrambled siRNA; right, Mff siRNA. Yellow arrows, independent punctae; blue arrow, peroxisome-associated puncta; white arrow, mitochondrially associated Mff. (B) Graph depicting the percentage of colocalization between independent Mff punctae and ER in U2OS cells (endogenous Mff). 54 independent Mff punctae were counted from five ROIs from four cells. Mean values from ROIs: 89.3 ± 6.7%, colocalized Mff with ER; 6.0 ± 6.1%, not colocalized; 4.8 ± 7.3%, unclear localization. (C) Live-cell time-lapse of GFP-Mff-S (green) in U2OS cell also expressing mCherry-mito3 (gray), eBFP2-peroxisome (blue), and E2-Crimson-ER (red). Yellow arrows, independent Mff punctae associating with ER; blue and gray arrows, peroxisomal and mitochondrial Mff, respectively. See also Video 6. (D) Graph depicting the degree of association between independent GFP-Mff-S punctae and ER from live-cell videos as in C (2.5-min videos imaged every 1.5 s). 34 ROIs from 30 U2OS cells analyzed (441 independent Mff punctae). Mean values from ROIs: 86.1 ± 17.1%, stably associated Mff punctae with ER; 11.0 ± 16.8%, partially associated; 4.6 ± 9.4%, not associated. (E) U2OS fractionation. Left: LSP, MSP, and HSP are low, medium, and high-speed pellets; HSS, high-speed supernatant. Marker proteins are ATP synthase, mitochondria; Sec63, ER; and Pmp70, peroxisomes. Right) Sucrose gradient fractionation of the medium-speed supernatant (MSS). (F) Human PEX3-deficient fibroblast fractionation, similar to U2OS fractionation. Bars: (B, whole cell image) 10 µm; (B, inset; and D) 2 µm. Time in seconds.