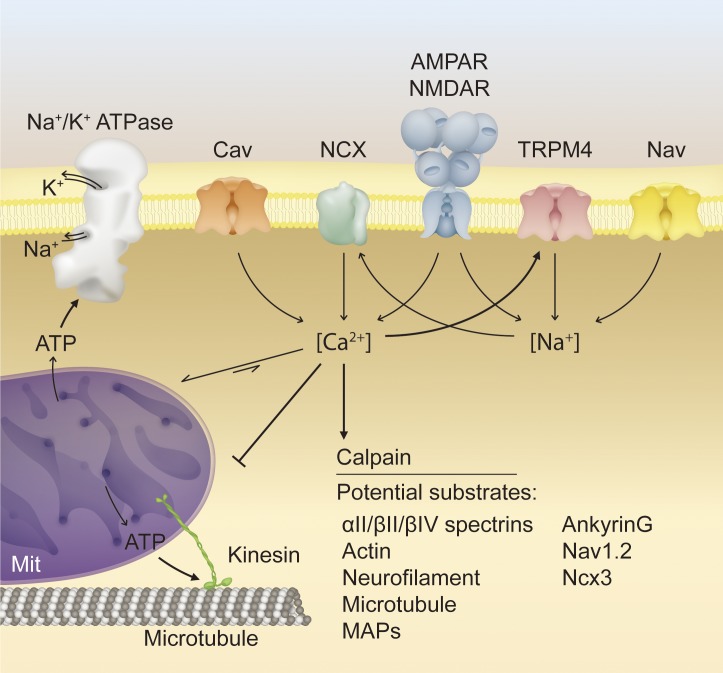
Figure 2.
Proposed molecular mechanisms of secondary axon degeneration. Multiple pathological mechanisms converge synergistically on axon degeneration. In a demyelinated axon, disassembly of nodal architecture and its underlying cytoskeleton may lead to voltage-gated sodium channel declustering, increasing sodium ion influx and the metabolic demand of action potential conduction. Impaired axon transport, increased metabolic load, and excitotoxic stress may lead to failure of the Na+/K+ ATPase, reversal of the NCX, and entry of calcium into the axon. Increased axoplasmic calcium leads to activation of the calcium-dependent protease calpain, which cleaves many putative substrates crucial to axon function, including those associated with the axon cytoskeleton or transport machinery. Overloading of mitochondrial calcium buffering capacity and impairment of mitochondrial transport via disruption of motor proteins and microtubules inhibits ATP production and exacerbates metabolic impairment. Excitotoxic stress further contributes to increased sodium and calcium influx, transmitted through various ion channels. NMDAR, N-Methyl-d-aspartate receptor. AMPAR, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor; Cav, voltage-gated calcium channel; MAP, microtubule-associated protein; Mit, mitochondria; Nav, voltage-gated sodium channel.