Abstract
The DOC-2/DAB2 interactive protein (DAB2IP) is a novel member of the Ras GTPase-activating protein family, and its downregulation is an unfavorable prognostic factor in several human malignancies. In this study, we retrospectively analyzed clinicopathological features, outcomes, and DAB2IP expression in 77 patients with urothelial carcinoma of the bladder (UCB). Expression of DAB2IP and p-STAT3 was examined in tumors and in matched adjacent non-cancerous tissues, using immunohistochemistry. We found a marked reduction in the expression of DAB2IP in UCB specimens, which was significantly associated with advanced pathological stage (P=0.037), high pathological grade (P=0.016), and muscle invasion (P=0.004). Moreover, multivariate analysis identified DAB2IP as an independent prognostic factor of cancer-specific survival (hazard ratio [HR] =0.23, 95% confidence interval [CI] =0.07–0.78, P=0.018). Most importantly, DAB2IP was negatively correlated with p-STAT3 expression (P=0.009), which predicted a shorter overall survival in patients with UCB (HR =2.68, 95% CI =1.63–6.99, P=0.044). In conclusion, downregulation of DAB2IP is associated with features of biologically aggressive UCB and may be a promising biomarker in patients after radical cystectomy.
Keywords: bladder urothelial carcinoma, DAB2IP, therapy targets, prognosis
Introduction
Bladder carcinoma is one of the most common urologic malignancies worldwide.1 Urothelial carcinoma of the bladder (UCB) accounts for 90% of all bladder carcinomas, and 70% of UCBs are non-muscle-invasive bladder cancers and the remaining 30% muscle-invasive bladder cancers.1,2 Despite advances in surgical techniques and treatments, the prognosis of UCB remains dismal.3 There is a high frequency of tumor recurrence after bladder preservation therapy, with a 5-year survival rate of <50%.3 As such, there is a great need to identify novel prognostic biomarkers and potential therapeutic targets to improve the clinical management of UCB. The DOC-2/DAB2 interactive protein (DAB2IP), a novel member of the Ras GTPase-activating protein (Ras-GAP) family, is often downregulated in human malignancies, including prostate, breast, lung, liver, pancreatic, and gastrointestinal cancers.4–8 The N-terminal domain of DAB2IP binds to DIP1/2, a Ras GTPase-activating protein, forming a unique protein complex with negative regulatory activity that modulates Ras-mediated signaling.9 DAB2IP has been implicated in cell proliferation, apoptosis, migration, and invasion by cancer cells, via inactivation of the Ras-ERK and Akt pathways, activation of the ASK1-JNK pathway, and inhibition of the epithelial–mesenchymal transition.9–12 Recent discoveries have indicated that low DAB2IP expression contributes to a poor prognosis in several types of cancers and collectively suggests that DAB2IP may function as a tumor suppressor protein.4–8
The role of DAB2IP has not, however, been determined in UCB. In this study, we investigated the expression of DAB2IP in UCB specimens and in adjacent non-cancerous tissues and assessed its prognostic value in patients treated by radical cystectomy with bilateral lymph node dissection. We determined that DAB2IP was negatively associated with the expression of phosphorylated signal transducer and activator of transcription 3 (p-STAT3) and explored the prognostic role of p-STAT3 in UCB.
Patients and methods
We retrospectively examined the records and collected the tissue blocks of 77 patients with UCB who underwent surgical treatment between January 2012 and December 2015 at Zhejiang Cancer Hospital, China. Tumor-adjacent noncancerous tissues (>2 cm) from additional 40 UCB cases were included as controls. None of the patients had a history of systemic chemotherapy or radiotherapy before surgery. Among them, 20 cases whose frozen tissue samples were available in the tissue bank of our hospital were reorganized into a cohort for further mRNA and protein extraction and analyzed. All cases had complete clinicopathological records and follow-up information, and all surgeries were performed by experienced surgeons from the Zhejiang Cancer Hospital. Clinicopathological factors included age, sex, tumor grade, T stage, tumor size, tumor number, nerve invasion, lymphovascular invasion, lymph node metastasis, and pathological stage. Follow-up visits were performed every 3 months for the first 2 years after surgery, every 6 months during the third year, and yearly thereafter.
Tumor grade was determined based on the 2004 World Health Organization/International Society of Urologic Pathology classification, and the pathological stage was assigned according to the 2010 American Joint Committee on Cancer (AJCC) TNM staging system, seventh edition. This study was approved by the institutional review board of Zhejiang Cancer Hospital, and all patients provided written informed consent before surgery.
Immunohistochemistry (IHC) and scoring
IHC was performed on 3 μm-thick sections using standard protocols and an automated IHC staining system (Dako Autostainer Plus; Dako Denmark A/S, Glostrup, Denmark) as described previously.7 The primary antibodies used were those targeting human DAB2IP (clone ab87811, diluted 1:400, rabbit IgG1; Abcam, Cambridge, UK) and p-STAT3 (sc-8059, diluted 1:500, mouse IgG1, Santa Cruz Biotechnology Inc., Dallas, TX, USA). Antigen retrieval was achieved using EDTA buffer (pH 9.0) in a pressure cooker for 4 min for DAB2IP or 20 min for p-STAT3. The stained tissue sections were reviewed and scored separately by two pathologists blinded to the clinical parameters. Discordant cases were discussed using a double-headed microscope to obtain a consensus classification.
The total immunostaining score for DAB2IP and p-STAT3 was calculated as the sum of the percentage of stained cells and the staining intensity, according to previously published reports.7,11 The percentage of stained cells was scored as 0 (0%), 1 (1%–25%), 2 (26%–50%), 3 (51%–75%), or 4 (>75%). The staining intensity was scored as 0 (no staining), 1 (weak staining), 2 (moderate staining), or 3 (strong staining). The staining of DAB2IP and p-STAT3 was determined as negative (low expression, a final staining score of <3) or positive (high expression, a final staining score of ≥3).
Western blotting
Proteins were extracted from tissues using NucleoSpin TriPrep kits (MACHEREY-NAGEL GmbH & Co. KG, Duren, Germany). The extracted proteins were separated by 12% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto polyvinylidene difluoride membranes (EMD Millipore, Billerica, MA, USA). Each membrane was blocked with 5% non-fat dry milk or bovine serum albumin in tris buffered saline-Tween 20 (TBST) for 1 h, followed by incubation with the appropriate primary antibody at 4°C overnight. After washing in TBST, the membranes were incubated with a horseradish peroxidase-conjugated secondary antibody at room temperature for 1 h and visualized by enhanced chemiluminescence (EMD Millipore).
RNA extraction and quantitative real-time polymerase chain reaction (PCR)
Total RNA was isolated from tissues using the RNeasy Mini kit (Qiagen NV, Venlo, the Netherlands), and 500 ng of each sample was reverse transcribed using the PrimeScript™ RT Reagent Kit (Takara Biotechnology Co., Ltd., Dalian, China). Quantitative PCR was performed to determine gene expression levels using SYBR® Premix Ex Taq™ II (Takara Biotechnology Co., Ltd.) on the ABI 7500 real-time PCR system (Thermo Fisher Scientific, Waltham, MA, USA). PCR conditions were 50°C for 2 min, followed by 40 cycles at 95°C for 5 s and 60°C for 35 s. The sequences of the PCR primers used were as follows: DAB2IP, 5′-CAGCAGTCCTCTTCCTCCAA-3′ (forward) and 5′-CTCGTCCTTACTCCTTAGCCTAT-3′ (reverse); STAT3, 5′-GAAGGACATCAGCGGTAAGAC-3′ (forward) and 5′-GATAGAGATAGACCAGTGGAGACA-3′ (reverse). Target gene expression was normalized to that of GAPDH and calculated as 2−ΔΔCt.
Statistical analyses
Statistical analyses were performed using SPSS software (version 18.0; SPSS Inc., Chicago, IL, USA). Associations of DAB2IP expression with clinicopathological variables were analyzed using Pearson’s chi-square or Fisher’s exact test. Spearman’s rank correlation coefficient was used to evaluate the correlation between DAB2IP and p-STAT3 expression. Associations of DAB2IP expression with overall survival (OS) were analyzed using the Kaplan–Meier method, log-rank test, and Cox proportional hazards model. P-values <0.05 in two-tailed tests were considered statistically significant.
Results
Downregulated DAB2IP expression in UCB specimens
DAB2IP expression profiles were determined in all of the UCB specimens and matched adjacent non-cancerous tissues (bladder urothelium controls) using IHC, real-time PCR, and Western blotting. IHC revealed a distinct expression pattern of DAB2IP in the UCB tissues that was not seen in the adjacent non-cancerous tissues. Of all UCB specimens, 68.8% (53/77) showed low expression of DAB2IP, compared with only 10.0% (4/40) of the bladder urothelium controls (P<0.05). Figure 1 shows representative images of DAB2IP-negative and DAB2IP-positive staining in UCB and adjacent non-cancerous tissues; staining of DAB2IP was noted in the cytoplasm of bladder urothelial cells. Furthermore, real-time PCR and Western blotting detected clear downregulation of DAB2IP mRNA (Figure 2, P<0.05) and protein expression in the 20 pairs of frozen UCB tissue samples and the related control tissues (Figure 3).
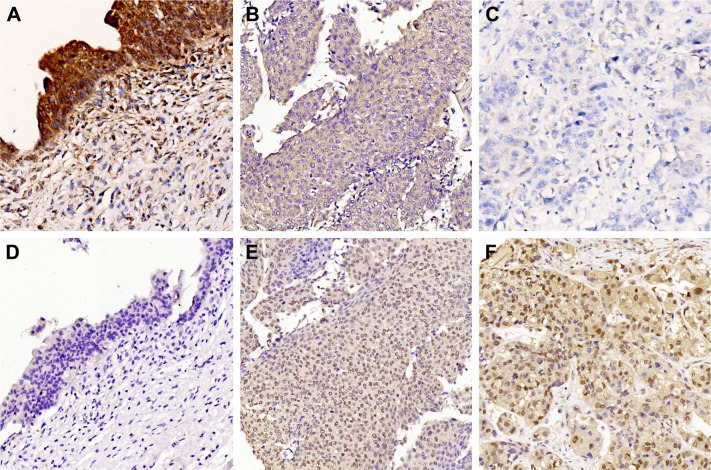
Figure 1.
Expression of DOC-2/DAB2 interactive protein (DAB2IP) and phosphorylation signal transducer and activator of transcription 3 (pSTAT3) in UCB tissues and bladder urothelium controls by immunohistochemistry.
Notes: (A) High expression of DAB2IP in normal bladder urothelium. (B) High expression of DAB2IP in low-grade UCB tissue. (C) Low expression of DAB2IP in high-grade UCB tissue. (D) Low expression of p-STAT3 in normal bladder urothelium. (E) High expression of p-STAT3 in low-grade UCB tissue. (F) High expression of p-STAT3 in high-grade UCB tissue. Magnification, 200×.
Abbreviation: UCB, urothelial carcinoma of the bladder.
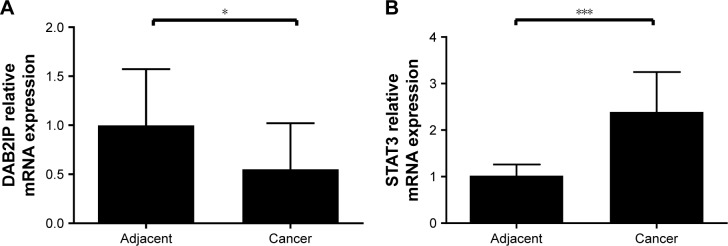
Figure 2.
Relative mRNA levels of DAB2IP and STAT3 in UCB and adjacent non-cancerous tissues.
Notes: (A) Real-time PCR showed that expression of DAB2IP was downregulated in cases of UCB compared with adjacent non-cancerous tissues (P<0.05). Results (mean ± SD) were obtained from three independent experiments. (B) Real-time PCR showed that expression of STAT3 was upregulated in cases of UCB compared with adjacent non-cancerous tissues (P<0.001). Results (mean ± SD) were obtained from three independent experiments. *P<0.05; ***P<0.001.
Abbreviations: PCR, polymerase chain reaction; UCB, urothelial carcinoma of the bladder.
Figure 3.
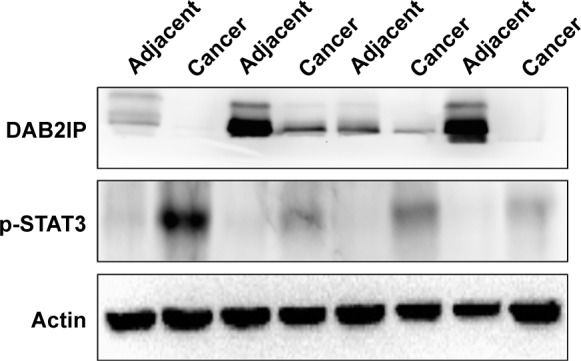
DAB2IP and p-STAT3 expressions in UCB and adjacent non-cancerous tissues.
Notes: Western blot results showed that expression of DAB2IP was downregulated in cases of UCB compared with adjacent non-cancerous tissues. Actin was presented as an internal loading control. Western blot results showed that expression of p-STAT3 was upregulated in cases of UCB compared with adjacent non-cancerous tissues. Actin was presented as an internal loading control. Results of 4 pairs in the total 20 pairs of samples are presented in this figure.
Abbreviation: UCB, urothelial carcinoma of the bladder.
Association of DAB2IP expression with the clinicopathological features of UCB
The associations of DAB2IP with the clinicopathological characteristics of UCB patients are summarized in Table 1. Among the 77 patients, 31 (40.3%) were younger than 65 years, and 70 (90.9%) were male. Regarding the tumor grade, 53/77 (68.8%) tumors were poorly differentiated, and 24/77 (31.2%) were well differentiated. According to the T stage in this cohort, 27 cases (35.1%) were non-muscle-invasive (Ta+T1), and 50 (64.9%) were muscle-invasive (T2+T3+T4). The tumor size was ≥2 cm in 59 (76.6%) cases, and 9 (11.7%) cases harbored multiple tumors. According to the AJCC TNM staging system, seventh edition, 41 cases (53.2%) were Stages 1–2 and 36 (46.8%) Stages 3–4. Statistical analyses indicated that the low expression of DAB2IP was significantly correlated with high tumor grade, muscle invasion, and advanced stage (P<0.05). No significant associations were found between DAB2IP expression and age, sex, tumor size, tumor number, nerve invasion, lymphovascular invasion, or nodal metastasis (P>0.05).
Table 1.
Association of DAB2IP expression with clinicopathological factors in UBC patients
| Category | All cases, n (%) | DAB2IP, n (%)
|
P-value | |
|---|---|---|---|---|
| Low | High | |||
| Age | 0.241 | |||
| <65 years | 31 (40.3) | 19 (61.3) | 12 (38.7) | |
| ≥65 years | 46 (59.7) | 34 (73.9) | 12 (26.1) | |
| Sex | 0.312 | |||
| Male | 70 (90.9) | 47 (67.1) | 23 (32.9) | |
| Female | 7 (9.1) | 6 (85.7) | 1 (14.3) | |
| Tumor grade | 0.016 | |||
| Low | 24 (31.2) | 12 (50.0) | 12 (50.0) | |
| High | 53 (68.8) | 41 (77.4) | 12 (22.6) | |
| Tumor stage | 0.004 | |||
| Ta+T1 | 27 (35.1) | 13 (48.1) | 14 (51.9) | |
| T2+T3+T4 | 50 (64.9) | 40 (80.0) | 10 (20.0) | |
| Tumor size | 0.821 | |||
| ≥2 cm | 59 (76.6) | 41 (69.5) | 18 (30.5) | |
| <2 cm | 18 (23.4) | 12 (66.7) | 6 (33.3) | |
| Tumor number | 0.093 | |||
| Single | 68 (88.3) | 49 (72.1) | 19 (27.9) | |
| Multiple | 9 (11.7) | 4 (44.4) | 5 (55.6) | |
| Nerve invasion | 0.070 | |||
| Yes | 20 (26.0) | 17 (85.0) | 3 (15.0) | |
| No | 57 (74.0) | 36 (63.2) | 21 (36.8) | |
| Lymphovascular invasion | 0.188 | |||
| Yes | 24 (68.8) | 19 (79.2) | 5 (20.8) | |
| No | 53 (31.2) | 34 (64.2) | 19 (35.8) | |
| Nodal metastasis | 0.050 | |||
| Yes | 17 (22.1) | 15 (88.2) | 2 (11.8) | |
| No | 60 (77.9) | 38 (63.3) | 22 (36.7) | |
| TNM stage | 0.037 | |||
| 0+1+2 | 41 (53.2) | 24 (58.5) | 17 (41.5) | |
| 3+4 | 36 (46.8) | 29 (80.6) | 7 (19.4) | |
| Total | 77 (100) | 53 (68.8) | 24 (31.2) | |
Note: Bold values are statistically significant (P<0.05).
Abbreviation: UBC, urothelial carcinoma of the bladder.
Increased p-STAT3 expression in UCB and its association with DAB2IP expression
A previous study of non-muscle-invasive bladder cancer showed that DAB2IP can regulate tumor recurrence via the STAT3 signaling pathway.13 Thus, further analyses were performed to examine the expression of p-STAT3 in UCB. Figure 1 shows representative images of p-STAT3-negative and p-STAT3-positive staining in UCB and adjacent noncancerous tissues; p-STAT3 expression was located in both the nucleus and cytoplasm of bladder urothelial cells. High expression of p-STAT3 was observed in 70.1% (54/77) of UCB samples but in only 7.5% (3/40) of matched adjacent non-cancerous tissue samples (P<0.05). There were also significant correlations between p-STAT3 expression and patient age, tumor differentiation, tumor (T) stage, and tumor number (P<0.05). Moreover, real-time PCR and Western blotting also indicated increased p-STAT3 expression in the UCB specimens compared with bladder urothelium controls (Figures 2B and 3). Most importantly, we found that the expression of DAB2IP was inversely associated with p-STAT3 expression in UCB (Table 2, P=0.009). Further correlation analyses showed similar results (r=−0.296, P=0.009).
Table 2.
Association of p-STAT3 expression with clinicopathological factors in UBC patients
| Category | All cases, n (%) | p-STAT3, n (%)
|
P-value | |
|---|---|---|---|---|
| Low | High | |||
| Age | 0.016 | |||
| <65 years | 31 (40.3) | 14 (45.2) | 17 (54.8) | |
| ≥65 years | 46 (59.7) | 9 (19.6) | 37 (80.4) | |
| Sex | 0.345 | |||
| Male | 70 (90.9) | 22 (31.4) | 48 (68.6) | |
| Female | 7 (9.1) | 1 (14.3) | 6 (85.7) | |
| Tumor grade | 0.009 | |||
| Low | 24 (31.2) | 12 (50.0) | 12 (50.0) | |
| High | 53 (68.8) | 11 (20.8) | 42 (79.2) | |
| Tumor stage | 0.010 | |||
| Ta+T1 | 27 (35.1) | 13 (48.1) | 14 (51.9) | |
| T2+T3+T4 | 50 (64.9) | 10 (20.0) | 40 (80.0) | |
| Tumor size | 0.162 | |||
| ≥2 cm | 59 (76.6) | 20 (33.9) | 39 (66.1) | |
| >2 cm | 18 (23.4) | 3 (16.7) | 15 (83.3) | |
| Tumor number | 0.010 | |||
| Single | 68 (88.3) | 17 (25.0) | 51 (75.0) | |
| Multiple | 9 (11.7) | 6 (66.7) | 3 (33.3) | |
| Nerve invasion | 0.580 | |||
| Yes | 20 (26.0) | 18 (31.6) | 39 (68.4) | |
| No | 57 (74.0) | 5 (25.0) | 15 (75.0) | |
| Lymphovascular invasion | 0.088 | |||
| Yes | 24 (31.2) | 4 (16.7) | 20 (83.3) | |
| No | 53 (68.8) | 19 (35.8) | 34 (64.2) | |
| Nodal metastasis | 0.212 | |||
| Yes | 17 (22.1) | 3 (17.6) | 14 (82.4) | |
| No | 60 (77.9) | 20 (33.3) | 40 (66.7) | |
| TNM stage | 0.707 | |||
| 0+1+2 | 41 (53.2) | 13 (31.7) | 28 (68.3) | |
| 3+4 | 36 (46.8) | 10 (27.8) | 26 (72.2) | |
| DAB2IP | 0.009 | |||
| Positive | 24 (31.2) | 12 (50.0) | 12 (50.0) | |
| Negative | 53 (68.8) | 11 (20.8) | 42 (79.2) | |
| Total | 77 (100) | 23 (29.9) | 54 (70.1) | |
Note: Bold values are statistically significant (P<0.05).
Abbreviation: UBC, urothelial carcinoma of the bladder.
Prognostic effects of DAB2IP and p-STAT3 expression in UCB
As shown in Table 3, a univariate Cox proportional hazards model demonstrated that patients with high DAB2IP expression had a lower risk of death than did those with low expression (hazard ratio [HR] =0.17, 95% confidence interval [CI] =0.05–0.56, P=0.003). Moreover, UCB patients who had non-muscle-invasive bladder cancer, absence of nodal metastasis, or early disease stage were more likely to show an extended survival (P<0.05, Figure 4). In multivariate analyses, when controlling for the effects of standard clinicopathological parameters, the expression of DAB2IP was found to be an independent prognostic factor of OS (HR =0.23, 95% CI =0.07–0.78, P=0.018). Pathological tumor stage was also an independent predictor of OS (HR =4.06, 95% CI =1.73–9.52, P=0.001). Kaplan–Meier analysis and log-rank tests yielded similar results (Figure 5A). The median OS of patients with high DAB2IP expression was significantly longer than that of those with low expression (not reached vs 21.0 months, P=0.001). Moreover, patients with p-STAT3 negativity had a significantly longer OS than did those with p-STAT3 positivity (median OS: not reached vs 28.0 months, P=0.033, Figure 5B). However, multivariate analysis failed to identify p-STAT3 as an independent prognostic factor for UCB (Table 3).
Table 3.
Univariate and multivariate Cox regression analyses estimating the associations of DAB2IP expression with patient survival
| Category | Crude HR | 95% CI | P-value | Adjusted HR | 95% CI | P-value |
|---|---|---|---|---|---|---|
| T stage | 0.005 | 0.827 | ||||
| Ta+T1 | 1 | 1 | ||||
| T2+T3+T4 | 4.53 | 1.58–13.0 | 0.83 | 0.18–3.85 | ||
| Nodal metastasis | <0.001 | 0.074 | ||||
| No | 1 | 1 | ||||
| Yes | 4.00 | 1.93–8.26 | 2.00 | 0.93–4.30 | ||
| TNM stage | <0.001 | 0.001 | ||||
| 0+1+2 | 1 | 1 | ||||
| 3+4 | 5.15 | 2.2–11.97 | 4.06 | 1.73–9.52 | ||
| DAB2IP | 0.003 | 0.018 | ||||
| Negative | 1 | 1 | ||||
| Positive | 0.17 | 0.05–0.56 | 0.23 | 0.07–0.78 |
Note: Bold values are statistically significant (P<0.05).
Abbreviations: CI, confidence interval; HR, hazard ratio.
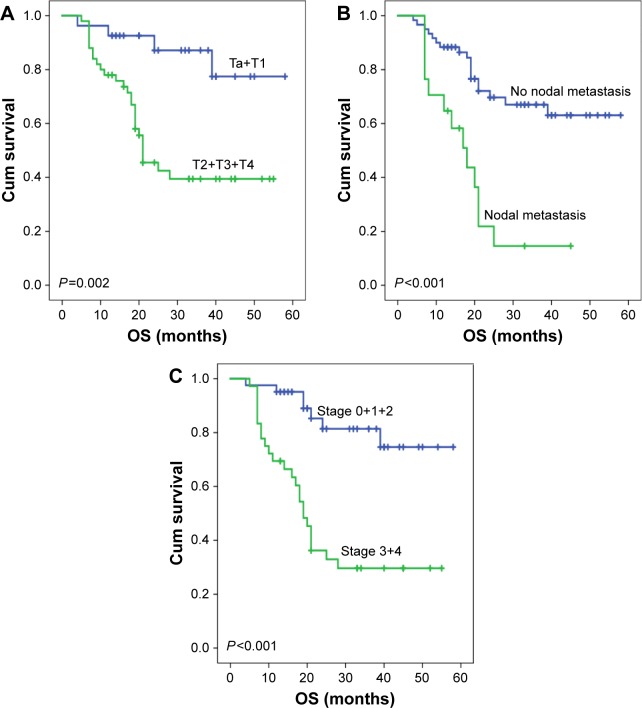
Figure 4.
Kaplan–Meier curves of OS in bladder cancer based upon T stage, nodal metastasis, and TNM stage.
Notes: (A) Patients with non-muscle-invasive bladder cancer (Ta+T1) had significantly longer OS than those with muscle-invasive bladder cancer (median OS: not reached vs 21.0 months, P=0.002). (B) There was statistically significant difference in OS between the patients with negative and positive nodal metastases (median OS: not reached vs 18.0 months, P<0.001). (C) Patients with early disease stage had significantly longer OS than those with advanced stage (median OS: not reached vs 19.0 months, P<0.001).
Abbreviations: Cum, cumulative; OS, overall survival.
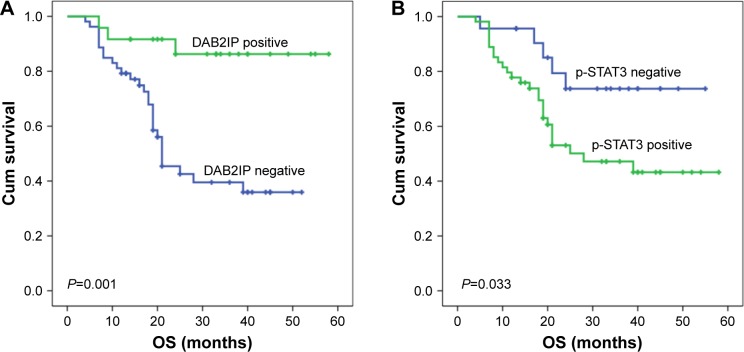
Figure 5.
Kaplan–Meier curves of OS in bladder cancer based upon DAB2IP and p-STAT3 expression.
Notes: (A) Patients with DAB2IP expression had significantly longer OS than those without DAB2IP expression (median OS: not reached vs 21.0 months, P=0.001). (B) There was statistically significant difference in OS between the patients with negative and positive p-STAT3 staining (median OS: not reached vs 28.0 months, P=0.033).
Abbreviations: Cum, cumulative; OS, overall survival.
Discussion
In the present study, we investigated the expression level and prognostic value of both DAB2IP and p-STAT3 in UCB. We found that DAB2IP was downregulated in UCB, which was strongly correlated with unfavorable tumor characteristics and that it acted as an independent prognostic factor of cancer-specific survival. We also observed an inverse association between DAB2IP and p-STAT3 expression in UCB. Moreover, patients with p-STAT3 positivity had a shorter OS than that of those negative for p-STAT3, although p-STAT3 expression failed to independently predict the outcome of UCB.
In this study, 68.8% of the UCB tissue samples showed low expression of DAB2IP, compared with only 10% of bladder urothelium controls. This was in line with a previous study of UCB,11 as well as with those of other cancers, including prostate cancer and hepatocellular carcinoma.7,14 Our analyses using real-time PCR and Western blotting also showed downregulated expression of DAB2IP in UCB specimens. In addition, and consistent with previous studies,11,15 we verified the association between decreased expression of DAB2IP and a high tumor grade, the presence of muscle invasion, and advanced tumor stage, suggesting that low expression of DAB2IP is a feature of aggressive tumors in UCB.
Tumor stage and grade are the most reliable prognostic markers known for bladder cancer.11 However, current pathological features are not sufficient for accurate prediction of recurrence and progression in patients with bladder cancer as a result of considerable tumor heterogeneity.13 The need for more molecular biomarkers has been recognized, and these may help refine the cancer risk stratification,16,17 identifying high-risk UCB patients and improving clinical management. Our study indicates that DAB2IP is a promising prognostic biomarker for UCB, in agreement with previous studies.7,8 After controlling for the effects of standard clinicopathological features, tumor stage and DAB2IP were found to be independent prognostic factors for OS, suggesting that measurement of DAB2IP expression is crucial in determining UCB patient prognosis and treatment options.
Low expression of DAB2IP was correlated with unfavorable tumor characteristics and outcomes in UCB. A previous study in prostate cancer indicated that DAB2IP loss leads to the activation of STAT3, which leads to inhibition of apoptosis.18 Moreover, in non-muscle-invasive bladder cancer, DAB2IP regulates tumor recurrence via STAT3/Twist1/P-glycoprotein signaling.13 Therefore, we speculated that DAB2IP is involved in regulating transcription via STAT3 in UCB. Importantly, we found that low expression of DAB2IP was significantly correlated with increased p-STAT3 expression in UCB. In prostate cancer, DAB2IP has been shown to bind directly to the unique proline-rich domain of STAT3, thereby inactivating it.18 Constitutive activation of STAT3 signaling plays an important role in bladder cancer cell growth, survival, invasion, and metastasis.19,20 Therefore, we speculate that DAB2IP might be involved in tumor initiation and progression via STAT3. However, the underlying mechanisms need further research.
Our study is not devoid of limitations, including those inherent to its retrospective design and the small cohort of cases from a single center, which may have introduced patient selection bias and/or changes in treatment strategies over time. Therefore, the predictive value of DAB2IP needs to be validated in prospective multicenter studies before clinical implementation.
Conclusion
The molecular and clinicopathological findings of this study highlight DAB2IP as a promising biomarker that predicts outcomes for UCB patients. Moreover, downregulated expression of DAB2IP was associated with high expression of p-STAT3 in UCB, the underlying mechanisms of which require further research.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Dettlaff K, Stawny M, Ogrodowczyk M, et al. Formulation and characterization of EGCG for the treatment of superficial bladder cancer. Int J Mol Med. 2017;40(2):329–336. doi: 10.3892/ijmm.2017.3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Babjuk M, Burger M, Zigeuner R, et al. European Association of Urology EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder: update 2013. Eur Urol. 2013;64(4):639–653. doi: 10.1016/j.eururo.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 4.Chen H, Toyooka S, Gazdar AF, Hsieh JT. Epigenetic regulation of a novel tumor suppressor gene (hDAB2IP) in prostate cancer cell lines. J Biol Chem. 2003;278(5):3121–3130. doi: 10.1074/jbc.M208230200. [DOI] [PubMed] [Google Scholar]
- 5.Dote H, Toyooka S, Tsukuda K, et al. Aberrant promoter methylation in human DAB2 interactive protein (hDAB2IP) gene in breast cancer. Clin Cancer Res. 2004;10(6):2082–2089. doi: 10.1158/1078-0432.ccr-03-0236. [DOI] [PubMed] [Google Scholar]
- 6.Yano M, Toyooka S, Tsukuda K, et al. Aberrant promoter methylation of human DAB2 interactive protein (hDAB2IP) gene in lung cancers. Int J Cancer. 2005;113(1):59–66. doi: 10.1002/ijc.20531. [DOI] [PubMed] [Google Scholar]
- 7.Zhang X, Li N, Li X, et al. Low expression of DAB2IP contributes to malignant development and poor prognosis in hepatocellular carcinoma. J Gastroenterol Hepatol. 2012;27(6):1117–1125. doi: 10.1111/j.1440-1746.2011.07049.x. [DOI] [PubMed] [Google Scholar]
- 8.Duan YF, Li DF, Liu YH, et al. Decreased expression of DAB2IP in pancreatic cancer with wild-type KRAS. Hepatobiliary Pancreat Dis Int. 2013;12(2):204–209. doi: 10.1016/s1499-3872(13)60032-6. [DOI] [PubMed] [Google Scholar]
- 9.Wang Z, Tseng CP, Pong RC, et al. The mechanism of growth-inhibitory effect of DOC-2/DAB2 in prostate cancer. Characterization of a novel GTPase-activating protein associated with N-terminal domain of DOC-2/DAB2. J Biol Chem. 2002;277(15):12622–12631. doi: 10.1074/jbc.M110568200. [DOI] [PubMed] [Google Scholar]
- 10.Xie D, Gore C, Liu J, et al. Role of DAB2IP in modulating epithelial-to-mesenchymal transition and prostate cancer metastasis. Proc Natl Acad Sci U S A. 2010;107(6):2485–2490. doi: 10.1073/pnas.0908133107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shen YJ, Kong ZL, Wan FN, et al. Downregulation of DAB2IP results in cell proliferation and invasion and contributes to unfavorable outcomes in bladder cancer. Cancer Sci. 2014;105(6):704–712. doi: 10.1111/cas.12407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xie D, Gore C, Zhou J, et al. DAB2IP coordinates both PI3K-Akt and ASK1 pathways for cell survival and apoptosis. Proc Natl Acad Sci U S A. 2009;106(47):19878–19883. doi: 10.1073/pnas.0908458106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu K, Wang B, Chen Y, et al. DAB2IP regulates the chemoresistance to pirarubicin and tumor recurrence of non-muscle invasive bladder cancer through STAT3/Twist1/P-glycoprotein signaling. Cell Signal. 2015;27(12):2515–2523. doi: 10.1016/j.cellsig.2015.09.014. [DOI] [PubMed] [Google Scholar]
- 14.Min J, Zaslavsky A, Fedele G, et al. An oncogene-tumor suppressor cascade drives metastatic prostate cancer by coordinately activating Ras and nuclear factor-kappaB. Nat Med. 2010;16(3):286–294. doi: 10.1038/nm.2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu JN, Wu KJ, Guan ZF, et al. DAB2IP expression in bladder transitional cell carcinoma and its correlation with clinical outcome. Sichuan Da Xue Xue Bao Yi Xue Ban. 2014;45(4):591–594. [PubMed] [Google Scholar]
- 16.Fristrup N, Ulhoi BP, Birkenkamp-Demtroder K, et al. Cathepsin E, maspin, Plk1, and survivin are promising prognostic protein markers for progression in non-muscle invasive bladder cancer. Am J Pathol. 2012;180(5):1824–1834. doi: 10.1016/j.ajpath.2012.01.023. [DOI] [PubMed] [Google Scholar]
- 17.Choi W, Porten S, Kim S, et al. Identification of distinct basal and luminal subtypes of muscle-invasive bladder cancer with different sensitivities to frontline chemotherapy. Cancer Cell. 2014;25(2):152–165. doi: 10.1016/j.ccr.2014.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou J, Ning Z, Wang B, et al. DAB2IP loss confers the resistance of prostate cancer to androgen deprivation therapy through activating STAT3 and inhibiting apoptosis. Cell Death Dis. 2015;6:e1955. doi: 10.1038/cddis.2015.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen CL, Cen L, Kohout J, et al. Signal transducer and activator of transcription 3 activation is associated with bladder cancer cell growth and survival. Mol Cancer. 2008;7:78. doi: 10.1186/1476-4598-7-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao D, Besser AH, Wander SA, et al. Cytoplasmic p27 promotes epithelial-mesenchymal transition and tumor metastasis via STAT3-mediated Twist1 upregulation. Oncogene. 2015;34(43):5447–5459. doi: 10.1038/onc.2014.473. [DOI] [PMC free article] [PubMed] [Google Scholar]