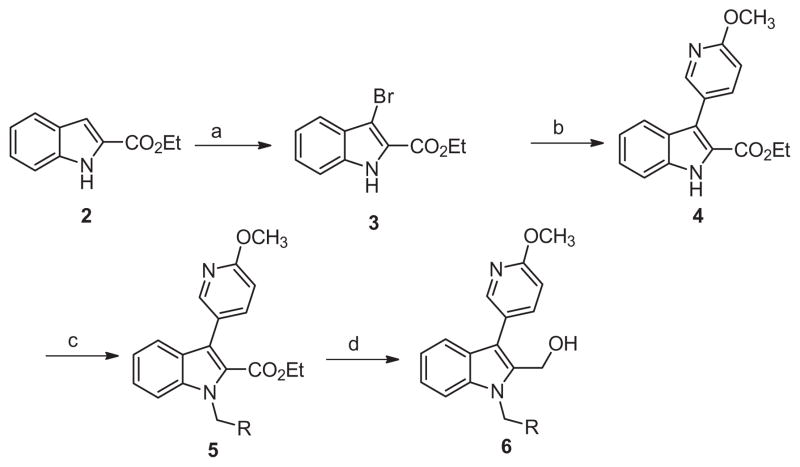
Scheme 1.
Reagents and conditions: (a) THF, NBS (1.0 equiv), rt, 2 h, 99%; (b) 6-methoxypyridin-3-yl phenylboronic acid (1.5 equiv), Pd(PPh3)4 (0.1 equiv), Na2CO3 (2.0 equiv), DMF–H2O (4:1), μw, 120 °C, 15 min, 75%; (c) K2CO3 (2 equiv), benzyl bromide (2 equiv, iodomethane applied for 6a), DMF, 45 °C, 16 h, 60–82%; (d) LiBH4, THF, rt, 16 h, 75–90%.