Table 2.
Silyl Aryl Iodide Reactionsa
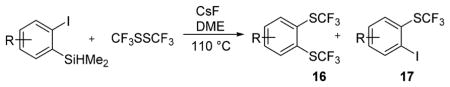
| |||
|---|---|---|---|
| entry | aryne precursor | 16 | yield (%) (16/17) |
| 1 |
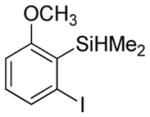
|
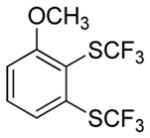
|
74 3.7:1.0 |
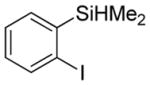
| 2 |
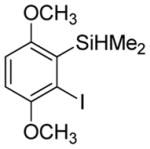
|
|
71b 3.1:1.0 |
| 3 |
|
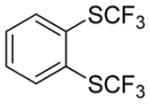
|
58c 2.4:1.0 |
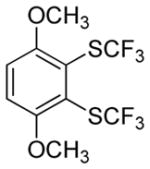
| 4 |
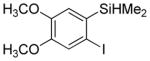
|
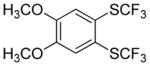
|
63 2.9:1.0 |
| 5 |
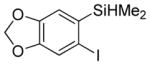
|
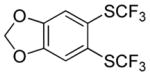
|
58 2.8:1.0 |
| 6 |
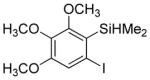
|
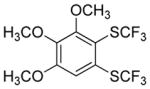
|
82 4.5:1.0 |
| 7 |
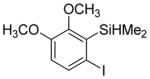
|
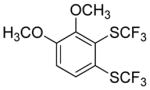
|
60 2.8:1.0 |
Reaction conditions: Aryne precursor (0.3 mmol), CsF (3 equiv), CF3SSCF3 (3.5 equiv), DME (2 mL), 37–46 h. Yields are isolated yields.
3,6-Dimethoxy-2-trifluoromethylthioiodobenzene (23%) also isolated.
Yield determined by NMR with PhCF3 as an internal standard. Please see Supporting Information for details.
