Abstract
Background
Ixodes ricinus is a species of hard tick that transmits several important diseases in Europe and North Africa, including Lyme borreliosis and tick-borne encephalitis. Climate change is affecting the geographic distributions and abundances of arthropod vectors, which in turn influence the geographic distribution and epidemiology of associated vector-borne diseases. To date, few studies have investigated effects of climate change on the spatial distribution of I. ricinus at continental extents. Here, we assessed the potential distribution of I. ricinus under current and future climate conditions to understand how climate change will influence the geographic distribution of this important tick vector in coming decades.
Method
We used ecological niche modeling to estimate the geographic distribution of I. ricinus with respect to current climate, and then assessed its future potential distribution under different climate change scenarios. This approach integrates occurrence records of I. ricinus with six relevant environmental variables over a continental extent that includes Europe, North Africa, and the Middle East. Future projections were based on climate data from 17 general circulation models (GCMs) under 2 representative concentration pathway emissions scenarios (RCPs), for the years 2050 and 2070.
Result
The present and future potential distributions of I. ricinus showed broad overlap across most of western and central Europe, and in more narrow zones in eastern and northern Europe, and North Africa. Potential expansions were observed in northern and eastern Europe. These results indicate that I. ricinus populations could emerge in areas in which they are currently lacking, posing increased risks to human health in those areas. However, the future of I. ricinus ticks in some important regions such the Mediterranean was unclear owing to high uncertainty in model predictions.
Introduction
Ixodes ricinus is the most common arthropod vector of human disease in Europe and nearby regions [1]. This tick species infests a wide variety of wild vertebrate species, as well as other accidental hosts, such as humans, livestock, and companion animals [2]. Ixodes ricinus transmits a wide variety of tick-borne pathogens, including the spirochete bacteria of the Borrelia burgdorferi sensu lato (sl) species complex and the tick-borne encephalitis virus. These pathogens are transmitted to humans mostly by bites of immature nymphs, which are more abundant, smaller and therefore harder to notice than adult ticks [3]. Some species of the B. burgdorferi sl complex cause Lyme disease (or Lyme borreliosis, LB) and tick-borne encephalitis virus causes tick-borne encephalitis (TBE). LB is prevalent in Central Europe where the highest infection rates have been recovered from ixodid ticks [4]. TBE occurs in eastern, western, and central Europe, as well as in Russia [5, 6]. Active surveillance of both diseases in Europe indicates a need to study the distributional ecology of their vector, I. ricinus. It also emphasizes the importance of understanding likely effects of global warming on the distribution of this vector species.
The geographic distribution of I. ricinus is related to climate factors such as humidity, soil water, and air temperature, and to vegetation type, land use, and disturbance [7]. Some studies have found that the latitudinal and elevational limits of I. ricinus have shifted with increasing global temperatures [8–10]. Over the last century, annual mean temperature has risen 0.7°C globally, and another 1.1°C increase is expected in the 21st century [11]. This warming is expected to influence vectors and reservoir hosts, in turn affecting the epidemiology of the vector-borne pathogens [11, 12]. Global warming is expected to alter vector development, vector physiology and fitness, geographic distribution of vectors and hosts, and vector-host-pathogen interactions [12]. Several recent publications have presented predictions of how climate change will alter the distribution of vector-borne diseases transmitted by ticks, sandflies, and mosquitoes. These studies investigate how changes in spatial distributions of arthropod vectors may influence future spatial distributions of vector-borne pathogens [13, 14].
Modeling the ecological requirements of species to anticipate future disease transmission patterns is challenging [11]. Previous studies of the potential distribution of I. ricinus have generally covered small geographic areas [15, 16], such as some studies in single countries that have attempted to understand the population dynamics of this species [17, 18]. A recent paper [19] studied effects of global climate change on I. ricinus across its range, but used older climate scenarios (4th generation Intergovernmental Panel on Climate Change (IPCC), emissions scenarios A2 and B2) from one general circulation model (GCM) only for projection. Here, we prepared a data set of I. ricinus occurrences that covered its entire geographic range in Europe and North Africa, and we carefully removed bias that might affect model predictions. We used a maximum entropy algorithm to estimate the ecological niche of I. ricinus, and transferred this model onto future conditions for the years 2050 and 2070 under 17 GCMs for two representative concentration pathway (RCP) scenarios for greenhouse gases. We thus present the most comprehensive models developed to date for this important disease vector, and explore their implications under the newest suite of future climate scenarios.
Materials and methods
Input data
Primary occurrence records for I. ricinus were obtained from diverse sources. Data were drawn from the Global Biodiversity Information Facility (GBIF; www.gbif.org; ~2110 occurrence points), VectorMap (www.vectormap.org; ~1801 occurrence points), and the scientific literature [20] (~1195 points; S1 File). Sampling was concentrated in Great Britain and Germany thanks to surveillance by the European Vector Map Program of the European Center for Disease Prevention and Control (ECDC; http://ecdc.europa.eu/en/healthtopics/vector/vector-maps/). The initial set of occurrence records was subjected to several data cleaning steps to reduce possible biases in calibrating ecological niche models (ENMs) [21]. (1) We discarded all records with unknown geographic references, and removed all duplicate records. (2) The data were further filtered by distance, so that all redundant records occurring in a single 10’ cell (~20 km) were omitted. (3) Finally, we accounted for marked differences in sampling density across countries: data records were filtered by balancing the density of occurrences on a country-by-country basis. We chose Spain as a reasonable intermediate-density reference point (6 occurrence records /100,000 km2) to overcome problems associated with oversampling or undersampling observed in some countries. Although we discarded large numbers of data points, this step removes large-scale spatial biases, and allows a better estimation of niche characteristics [22].
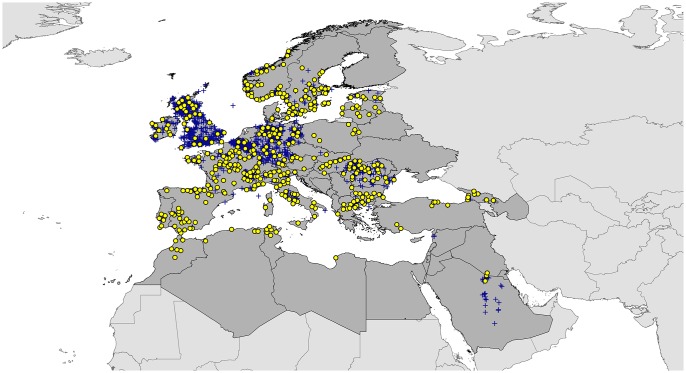
The final balanced dataset of I. ricinus included 416 occurrence points, which we separated five times randomly into equal-sized subsets of 208 points, one subset was for model calibration and the other for model evaluation (Fig 1). These 5 random subgroups provide replicate views of model results and give a better idea of the variation resulting from the availability of occurrence data.
Fig 1. Occurrence records of Ixodes ricinus derived from various sources.
Blue crosses indicate the original set of occurrence records; yellow circles are occurrence records retained after filtering the data.
We obtained data on 19 “bioclimatic” variables from the WorldClim climate data version 1.4 [23] available via www.worldclim.org. These variables were derived from interpolation of average monthly temperature and rainfall data obtained from weather stations during 1950–2000. We removed variables 8–9 and 18–19 because of known spatial artefacts. We used the data layers at 10’ spatial resolution because of the continental extent of our models. We obtained parallel data layers for 17 general circulation models (GCMs; Table 1) for each representative concentration pathway (RCP) for each time period. We chose two representative concentration pathways, RCP 4.5 and RCP 8.5 (corresponding to lower and higher greenhouse gas emissions, respectively) for 2050 and 2070 to account for possible climate change influences in both scenarios and in two different times. We used diverse GCMs available from the WorldClim archive to estimate both the future distributional potential of I. ricinus based on each individual GCM, which was a key element in assessing uncertainty in predictions deriving from GCM choice.
Table 1. Summary of general circulation models (GCMs) explored in our analysis.
| GCM | Code | Modeling center or group |
|---|---|---|
| ACCESS 1–0 | AC | Commonwealth Scientific and Industrial Research Organization (CSIRO) and Bureau of Meteorology (BOM), Australia |
| BCC-CSM 1–1 | BC | Beijing Climate Center, China Meteorological Administration |
| CCSM4 | CC | National Center for Atmospheric Research, USA |
| CNRM-CM 5 | CN | Centre National de Recherches Météorologiques, France |
| GFDL-CM 3 | GF | NOAA Geophysical Fluid Dynamics Laboratory, USA |
| GISS-E2-R | GS | NASA Goddard Institute for Space Studies, USA |
| HadGEM 2-AO | HD | National Institute of Meteorological Research, Korea Meteorological Administration |
| HadGEM 2-ES | HE | Met Office Hadley Centre (additional realizations from Instituto Nacional de Pesquisas Espaciais) |
| HadGEM 2-CC | HG | Met Office Hadley Centre (additional realizations from Instituto Nacional de Pesquisas Espaciais). |
| INMCM4 | IN | Institute for Numerical Mathematics, Russia |
| IPSL-CM5A-LR | IP | Institute Pierre-Simon Laplace, France |
| MIROC-ESM-CHEM | MI | Japan Agency for Marine-Earth Science and Technology, Atmosphere and Ocean Research Institute, and National Institute for Environmental Studies, Japan |
| MIROC-ESM | MR | Japan Agency for Marine-Earth Science and Technology, Atmosphere and Ocean Research Institute, and National Institute for Environmental Studies, Japan |
| MIROC5 | MC | Atmosphere and Ocean Research Institute, National Institute for Environmental Studies, and Japan Agency for Marine-Earth Science and Technology, Japan |
| MPI-ESM-LR | MP | Max-Planck-Institut für Meteorolgie, Germany |
| MRI-CGCM3 | MG | Meteorological Research Institute, Japan |
| NorESM 1-M | NO | Norwegian Climate Centre, Norway |
Ecological niche modeling
Ecological niche models (ENMs) were estimated based on the maximum entropy algorithm implemented in Maxent 3.3.3k [24]. We first used the jackknifing function in Maxent to identify the most important set of environmental variables. We used SDMTools in ArcGIS 10.3 to remove variables with high inter-variable correlations. In the end, we used 6 variables for analysis: (1) annual mean temperature, (2) mean diurnal temperature range, (3) isothermality, (4) annual temperature range, (5) annual precipitation, and (6) precipitation seasonality. Associations between presence points and environmental variables [25] were used to reconstruct the ecological niche of I. ricinus. We hypothesized an accessible area (M in the BAM diagram framework) [26] that included all of Europe, North Africa, and parts of the Middle East but excluded western Asia for lack of data documenting I. ricinus occurrence in this region. We used Maxent’s bootstrap function to create 10 replicate analyses. We used a partial receiver operating characteristic (pROC) [27] to test model robustness via Niche Toolbox (http://shiny.conabio.gob.mx:3838/nichetoolb2/); the 5 testing subsets of the occurrence data were used to test model predictions. For further evaluation of our predictions regarding the occurrence of I. ricinus, we used a set of 3186 I. ricinus records discarded during the early phases of data filtering of the original dataset. We excluded those records used in model calibration; we used a one-tailed cumulative binomial probability test to assess the probability of obtaining the observed level of correct predictions by chance alone given the background expectation of correct predictions determined by the proportional coverage of the study area by regions of predicted suitability.
To summarize the model results for present-day conditions, we calculated the median of the medians from the predictions based on the 5 subsets of occurrences as an estimate of the current geographic distribution of I. ricinus. For future conditions, we calculated medians across all single-GCM median model outputs (5 subsets of occurrences x 17 GCMs = 85 combinations), as an estimate of the potential distribution of I. ricinus under each corresponding RCP. For present-day conditions, an uncertainty index was derived from the range (maximum—minimum) of predictions from 5 Maxent runs (i.e. 5 combinations based on the 5 subsets of occurrence records), which summarizes uncertainty deriving from the particular availability of occurrence data. For the future conditions, an uncertainty index of future model predictions was calculated as the range across all combinations of GCMs and occurrence subsets (within each RCP; 5 subsets of occurrences x 17 GCMs = 85 combinations) [14], thereby including uncertainty deriving both from the availability of occurrence data and variation among GCMs in future climates anticipated. To avoid known problems with model overfitting [28], we thresholded models using a fixed allowable omission error rate of E = 5% [29], assuming that 5% of the occurrence data may have included errors that misrepresented environmental values. This thresholding approach omitted the 5% of records with the lowest suitability to account for the reality that such large datasets often include some errors (either in the occurrence data or the environmental data), so this 5% trimming allows those errors to be ignored and not affect the results.
Mobility-oriented parity (MOP) was used to assess the degree of novelty of climate conditions under all future climate scenarios (i.e., 17 GCMs x 2 RCPs x 2 time periods) relative to present-day conditions. This novelty is actually a distance in environmental space between the environmental characteristics of the site in question and the set of environments represented across the reference region. In the present study, the site in question is generally in a future time period, and the reference region is in the present. MOP evaluates the general novelty of conditions, and highlights regions where strict extrapolation (i.e. values outside of the range of environments in the reference region/time) occurs, to give a view of certainty and uncertainty across various sectors of the region of interest [30]. Any extrapolative transfer of the model should be interpreted with considerable caution. To summarize MOP results within each RCP, we counted the number of GCMs for which each pixel was strictly extrapolative, and also calculated the average distance from the reference (present) conditions.
Results
After detailed data cleaning and spatial balancing, our initial total of 5107 occurrence points for I. ricinus from diverse sources was filtered and reduced to 416 spatially unique points at 10’ resolution. This data filtering reduced problems associated with artificial clumping of occurrence records, which is related to biases in sampling and reporting (Fig 1). Calibrating models for I. ricinus based on the 5 subgroups of occurrence points yielded predictions that gave area under the curve (AUC) ratios above null expectations in all five partial ROC analyses (P < 0.001; S2 File). The I. ricinus model successfully anticipated 3110 records out of the additional 3186 test records, which was significantly better than random expectations (P < 0.001; S3 File). Annual precipitation, annual temperature range, and annual mean temperature were the most influential factors and contributed >86% to the Maxent model (S4 File).
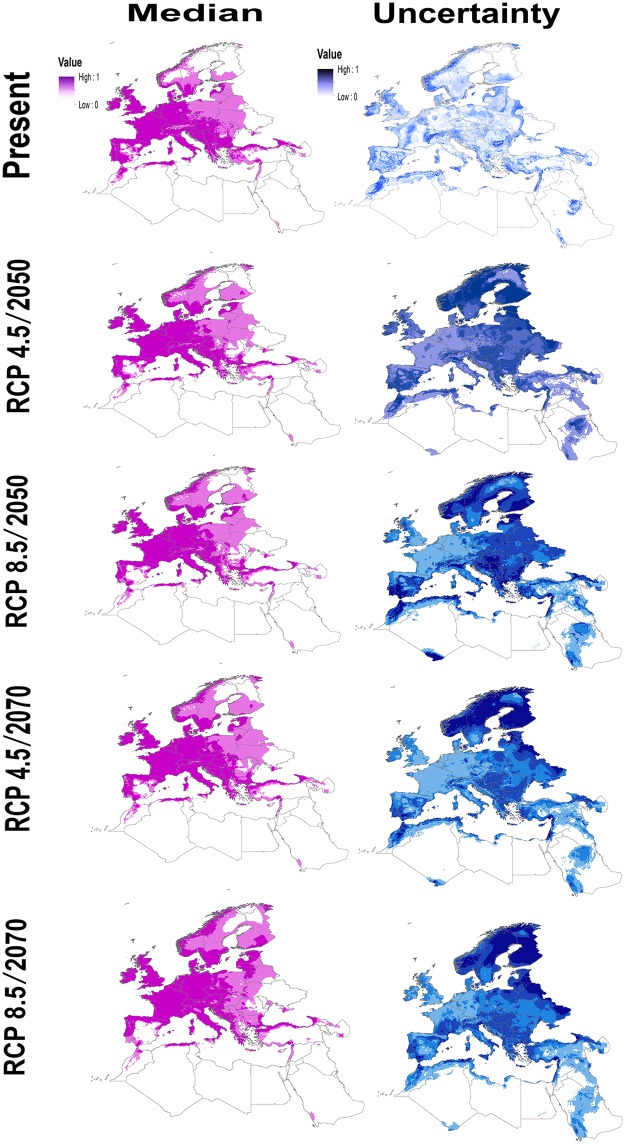
Models based on present-day conditions revealed areas with high suitability for I. ricinus across Central and Western Europe including the countries of Great Britain, France, Germany, Belgium, the Netherlands, Greece, and Italy. In Northern Europe, the highest suitability for I. ricinus was in southern Finland, southern Sweden, and western Norway. Suitable areas also occurred in western Turkey, the Middle East, and in more restricted areas in Morocco, Algeria, and Tunisia. Transferring models to future conditions, the present-day and future distributional patterns largely coincided. However, our model predictions indicated some potential for expansion into areas not identified as suitable for I. ricinus under present-day conditions, particularly in Northern Europe (Fig 2), albeit with high uncertainty with respect to the potential distributions in both present and future.
Fig 2. Current and future potential distribution of Ixodes ricinus based on present-day and future climatic conditions.
Left-hand maps show potential distributions whereas right-hand maps indicate the uncertainty.
Under present-day conditions, low uncertainty (deriving from availability of occurrence data) was observed across the study area (which can be uncertainty as regards prediction of presence or prediction of absence), except some areas in Morocco, Ireland, and eastern Norway (Fig 2). In the future, uncertainty with respect to predictions of geographic distribution of I. ricinus and deriving from both availability of occurrence data and variation among GCMs in future conditions anticipated was observed in Scandinavia and parts of Eastern Europe. Under future conditions, the levels of uncertainty varied among RCP scenarios and time periods, but this variation was not necessarily related to the density of the available occurrence data. For RCP 4.5 in 2050, high uncertainty was concentrated in southern Finland, central Norway, and Sweden, and medium uncertainty was present in parts of Eastern Europe and North Africa, whereas lower uncertainty was in Western Europe and the Middle East. For RCP 8.5 for the 2050s, high uncertainty was restricted to southern Finland, eastern Sweden, southern Spain, and northern Morocco; low uncertainty areas were in Western Europe and the Middle East. For RCP 4.5 for the 2070s, models showed high uncertainty in Finland, Norway, central and northern Sweden, and eastern Belarus. RCP 8.5 for the 2070s showed high uncertainty in Finland, eastern Sweden, eastern Belarus, and eastern Ukraine; low uncertainty was in Western Europe, North Africa, and the Middle East (Fig 2).
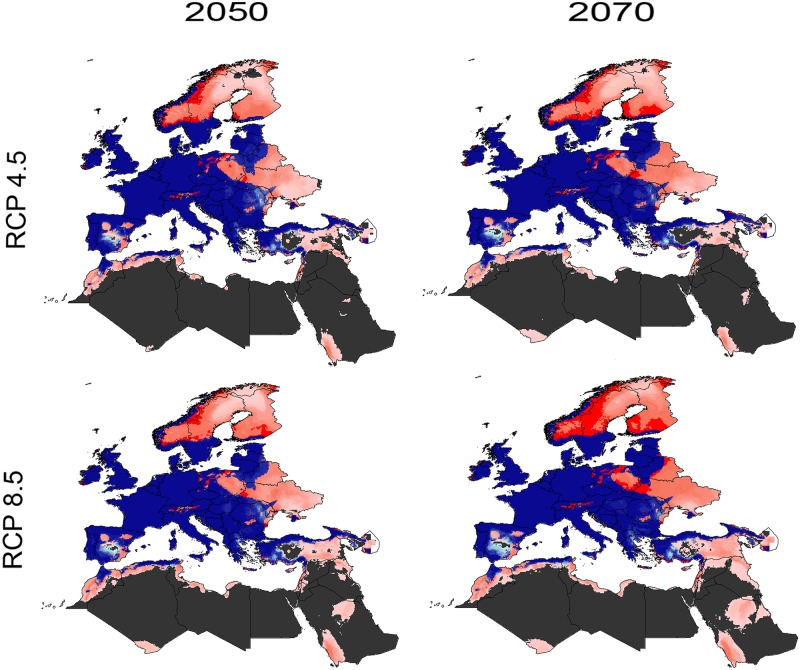
Binary (thresholded) predictions for future conditions showed differences between RCP 4.5 and RCP 8.5 in 2050 and 2070 (Fig 3). In terms of agreement between present and future conditions, range expansion was indicated in North Africa in the coastal regions of Morocco, Algeria, and Tunisia. Under RCP 4.5 for the 2050s, expansions are expected (although with low confidence) in North Africa, the Middle East, and Eastern and Northern Europe. Under RCP 8.5 for the 2050, the expansions in Eastern and Northern Europe, Turkey, and the Middle East were predicted to be wider compared to RCP 4.5 for 2050. In 2070, suitable areas for I. ricinus increased in Norway, Sweden, and Finland, with high confidence in Turkey, the Middle East and North Africa under RCP 4.5 and RCP 8.5 (Fig 3). Between the present-day and 2050, the potential distribution area of I. ricinus increased by 10.8% and 11.7% under RCP 4.5, and RCP 8.5, respectively. Between the present day and 2070, the potential distributional area of I. ricinus is anticipated to increase by 11.5% and 14.5% under RCP 4.5, and RCP 8.5, respectively. Thus, global warming is expected to increase the geographic distribution of I. ricinus in Europe, North Africa, and the Middle East. Compared to RCP 4.5, the potential distributional area under RCP 8.5 increased by 0.9% and 3.0% for 2050 and 2070, respectively; a summary matrix (S5 File) and detailed maps of potential distributions under each individual GCM are provided in the supplementary materials (S6 File); the final future projections are summarized as a GeoTIFF data file at https://doi.org/10.6084/m9.figshare.5067373.
Fig 3. Summary of the binary modeled potential distributions of Ixodes ricinus under future conditions to show suitable areas and to illustrate differences between representative concentration pathways (RCPs) and time periods.
Blue color indicates model suitability under both present and future suitability (light blue denotes low confidence and dark blue denotes high confidence), red color represents predicted expansion areas in the future suitability (light red = low confidence, dark red = high confidence); dark gray areas are not suitable.
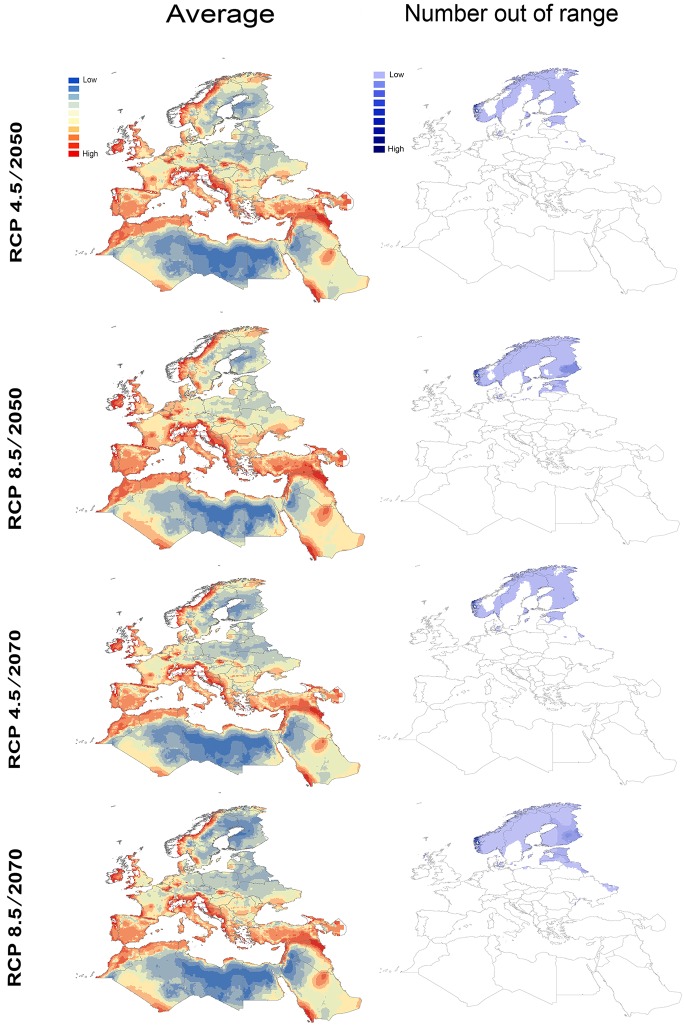
MOP results indicated high novelty of future conditions along the entire Mediterranean rim of southern Europe and North Africa, as well as in northern Scandinavia (Fig 4). Under RCP 4.5, MOP detected extrapolative conditions in as many as 10 of 17 models in both 2050 and 2070. Under RCP 8.5, MOP detected extrapolative conditions in 11 and 13 of 17 models in 2050 and 2070, respectively. Strict extrapolation was manifested chiefly in northern extremes of Scandinavian countries.
Fig 4. MOP calculations for model transfers from present to future climate scenarios for 17 GCMs (RCP 4.5 and RCP 8.5) in 2050 and 2070.
Left-hand panels show the average MOP distance among models (dark red represents high average and dark blue represents low average). Right-hand panels show the number of models out of range (dark blue represents areas with most frequent strict extrapolation).
Discussion
Tick-borne diseases such as TBE and LB are greatly influenced by tick ecology and other factors such as habitat structure, climate, human activities, and the community of vertebrate species that are reservoir hosts for the tick-borne pathogens [31]. Several studies have suggested that increasing temperatures will affect the geographic distribution and ecology of I. ricinus in Europe [19, 32, 33]. Climate change may affect the seasonal activities and feeding behavior of the different life stages of I. ricinus [34, 35]. For instance, rising temperatures could lead to milder winter conditions, extending spring and fall seasons in northern regions, making them more suitable for I. ricinus. Indeed, expansions of northern distributional limits have been reported for this species in Norway and Sweden since the 1980s [8, 36, 37]. Elevational range expansions of I. ricinus have been recorded in the Czech Republic and Switzerland [38]. Our results also showed that the presence of I. ricinus will increase in the European Alps with high certainty. Increased TBE transmission in Europe during the last 2 decades has been attributed to climate change, socio-economic changes, and anthropogenic activities [39].
Several factors must be considered before interpreting our model predictions regarding the expansions and changes in the potential distribution of I. ricinus. First, as with all ixodid ticks, I. ricinus spends most of its life cycle off the host and in the environment, so climate change may have direct effects on its abundance and distribution [40]. Second, other abiotic factors (e.g. land use, soil characteristics), and biotic factors (e.g., host abundance and competition with other species), should be considered in tandem with climate effects [40–44]. Third, newly suitable areas must be accessible to I. ricinus via dispersal for actual range expansions to take place [45]. For example, migratory birds are potential agents of tick dispersal across new regions [46]. Migratory bird-mediated dispersal may allow I. ricinus to respond to improving conditions in some of the expansion areas, and create new biotic combinations suitable for circulation of tick-borne pathogens.
Our results were similar to those of Porretta et al. [19] in terms of the distribution of I. ricinus presently extending across most of Europe and parts of North Africa and the Middle East. The two studies made similar future projections for I. ricinus: both anticipated potential for expansion into new areas in northern and eastern Europe. This study differs from Porretta et al. [19] in using 6 climate variables for analysis, the most updated RCPs, many more individual GCMs, and the IPCC 5th Assessment scenarios for 2050 and 2070. Also, we used diverse data sources, and focused on Europe, North Africa, and the Middle East, to develop predictions of the potential distribution of this tick species into the future. We did not include western Asia in our study owing to a lack of sufficient occurrence data from this region. In addition, we included mobility-oriented parity (MOP) to understand certainty and uncertainty in different areas in the region of interest [30]. Thus, whereas the results of the two studies did not differ qualitatively, our work provides a clearer picture of certainty and uncertainty in these predictions for an important disease vector species.
Our models anticipated potential range expansions more broadly in northern Europe, with milder winter conditions as temperature increases [19]. In Sweden, for example, the climate has changed to be significantly warmer in the last 3 decades: the 8 warmest Novembers on record were between 2000 and 2009 [47]. These changes can allow more ticks to survive the winter, and increase the probability of tick bites [19]. Given that LB, TBE, and various other tick-borne diseases cause serious health problems, predicting future suitable areas for I. ricinus can help to guide plans to manage and mitigate effects of these public health threats [48].
Supporting information
These records were drawn from the Global Biodiversity Information Facility (GBIF), VectorMap, and the scientific literature.
(CSV)
This supporting information describes the detailed results of model evaluation based on partial ROC tests applied to 5 random subsets of occurrence data.
(PDF)
The testing records are those retained from the original occurrences (i.e. black dots) but none coincided with the 416 records used in model calibration (i.e. yellow circles).
(PDF)
This supporting information shows the results regarding the relative contributions of six environmental variables to models for I. ricinus based on 5 random subsets of occurrences.
(PDF)
(CSV)
(PDF)
Acknowledgments
The authors would like to thank the University of Kansas Ecological Niche Modeling (KU ENM) Group, and the Surveillance, Ecology, and Epidemiology Research Group, Cairo, Egypt. Thanks to the Ministry of Higher Education, Libya, for supporting AAA during his studies at the University of Kansas; AMS was funded by the Egyptian Fulbright Mission Program [EFMP]. Special thanks to the staff of the Zoology Department, Faculty of Science, University of Tripoli, Tripoli, Libya; for their continuous support during the study. Thanks to Gengping Zhu and Katie Allen for assistance with data analysis. Thanks to Arwa Elaagip who hosted AMS during his work on this project at Khartoum University, Sudan.
Data Availability
All relevant data are within the paper and its Supporting Information files. GeoTIFF dataset for different general circulation models are openly available via Figshare repository (https://doi.org/10.6084/m9.figshare.5067373).
Funding Statement
AAA was supported by the Ministry of Higher Education, Libya. AMS was supported by the Graduate Fulbright Egyptian Mission Program (EFMP). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Parola P, Raoult D. Tick-borne bacterial diseases emerging in Europe. Clinical Microbiology and Infection. 2001;7(2):80–3. [DOI] [PubMed] [Google Scholar]
- 2.Estrada-Peña A, de la Fuente J. The ecology of ticks and epidemiology of tick-borne viral diseases. Antiviral Research. 2014;108:104–28. doi: 10.1016/j.antiviral.2014.05.016 [DOI] [PubMed] [Google Scholar]
- 3.Diuk-Wasser MA, Hoen AG, Cislo P, Brinkerhoff R, Hamer SA, Rowland M, et al. Human risk of infection with Borrelia burgdorferi, the Lyme disease agent, in eastern United States. The American journal of Tropical Medicine and Hygiene. 2012;86(2):320–7. doi: 10.4269/ajtmh.2012.11-0395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Derdáková M, Lencakova D. Association of genetic variability within the Borrelia burgdorferi sensu lato with the ecology, epidemiology of Lyme borreliosis in Europe. Annals of Agricultural and Environmental Medicine. 2005;12(2):165 [PubMed] [Google Scholar]
- 5.Hayasaka D, Ivanov L, Leonova GN, Goto A, Yoshii K, Mizutani T, et al. Distribution and characterization of tick-borne encephalitis viruses from Siberia and far-eastern Asia. Journal of General Virology. 2001;82(6):1319–28. [DOI] [PubMed] [Google Scholar]
- 6.Rauter C, Hartung T. Prevalence of Borrelia burgdorferi sensu lato genospecies in Ixodes ricinus ticks in Europe: a metaanalysis. Applied and Environmental Microbiology. 2005;71(11):7203–16. doi: 10.1128/AEM.71.11.7203-7216.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jore S, Vanwambeke SO, Viljugrein H, Isaksen K, Kristoffersen AB, Woldehiwet Z, et al. Climate and environmental change drives Ixodes ricinus geographical expansion at the northern range margin. Parasites & Vectors. 2014;7(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lindgren E, Tälleklint L, Polfeldt T. Impact of climatic change on the northern latitude limit and population density of the disease-transmitting European tick Ixodes ricinus. Environmental Health Perspectives. 2000;108(2):119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Danielová V, Schwarzová L, Materna J, Daniel M, Metelka L, Holubová J, et al. Tick-borne encephalitis virus expansion to higher altitudes correlated with climate warming. International Journal of Medical Microbiology. 2008;298:68–72. [Google Scholar]
- 10.Zeman P, Beneš C. A tick-borne encephalitis ceiling in Central Europe has moved upwards during the last 30 years: possible impact of global warming? International Journal of Medical Microbiology Supplements. 2004;293:48–54. [DOI] [PubMed] [Google Scholar]
- 11.Patz JA, Graczyk TK, Geller N, Vittor AY. Effects of environmental change on emerging parasitic diseases. International Journal of Parasitology. 2000;30(12):1395–405. [DOI] [PubMed] [Google Scholar]
- 12.Parham PE, Waldock J, Christophides GK, Hemming D, Agusto F, Evans KJ, et al. Climate, environmental and socio-economic change: weighing up the balance in vector-borne disease transmission. Phil Trans R Soc B. 2015;370(1665):20130551 doi: 10.1098/rstb.2013.0551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Medlock JM, Leach SA. Effect of climate change on vector-borne disease risk in the UK. Lancet Infectious Diseases. 2015;15(6):721–30. doi: 10.1016/S1473-3099(15)70091-5 [DOI] [PubMed] [Google Scholar]
- 14.Samy AM, Elaagip AH, Kenawy MA, Ayres CF, Peterson AT, Soliman DE. Climate change influences on the global potential distribution of the mosquito Culex quinquefasciatus, vector of West Nile virus and lymphatic filariasis. PLoS ONE. 2016;11(10):e0163863 doi: 10.1371/journal.pone.0163863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Estrada-Peña A, Venzal JM. Climate niches of tick species in the Mediterranean region: modeling of occurrence data, distributional constraints, and impact of climate change. Journal of Medical Entomology. 2007;44(6):1130–8. [DOI] [PubMed] [Google Scholar]
- 16.Medlock JM, Hansford KM, Bormane A, Derdakova M, Estrada-Peña A, George J-C, et al. Driving forces for changes in geographical distribution of Ixodes ricinus ticks in Europe. Parasites & Vectors. 2013;6(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dobson AD, Randolph SE. Modelling the effects of recent changes in climate, host density and acaricide treatments on population dynamics of Ixodes ricinus in the UK. Journal of Applied Ecology. 2011;48(4):1029–37. [Google Scholar]
- 18.Schwarz A, Maier WA, Kistemann T, Kampen H. Analysis of the distribution of the tick Ixodes ricinus L. (Acari: Ixodidae) in a nature reserve of western Germany using geographic information systems. International Journal of Hygiene and Environmental Health. 2009;212(1):87–96. doi: 10.1016/j.ijheh.2007.12.001 [DOI] [PubMed] [Google Scholar]
- 19.Porretta D, Mastrantonio V, Amendolia S, Gaiarsa S, Epis S, Genchi C, et al. Effects of global changes on the climatic niche of the tick Ixodes ricinus inferred by species distribution modelling. Parasites & Vectors. 2013;6(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Estrada-Peña A, Alexander N, Wint GW. Perspectives on modelling the distribution of ticks for large areas: so far so good? Parasites & Vectors. 2016;9(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Syfert MM, Smith MJ, Coomes DA. The effects of sampling bias and model complexity on the predictive performance of MaxEnt species distribution models. PLoS ONE. 2013;8(2):e55158 doi: 10.1371/journal.pone.0055158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fourcade Y, Engler JO, Rödder D, Secondi J. Mapping species distributions with MAXENT using a geographically biased sample of presence data: a performance assessment of methods for correcting sampling bias. PloS one. 2014;9(5):e97122 doi: 10.1371/journal.pone.0097122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A. Very high resolution interpolated climate surfaces for global land areas. International journal of climatology. 2005;25(15):1965–78. [Google Scholar]
- 24.Phillips SJ, Dudík M. Modeling of species distributions with Maxent: new extensions and a comprehensive evaluation. Ecography. 2008;31(2):161–75. [Google Scholar]
- 25.Phillips SJ, Anderson RP, Schapire RE. Maximum entropy modeling of species geographic distributions. Ecological Modelling. 2006;190(3):231–59. [Google Scholar]
- 26.Soberón J, Peterson A. Interpretation of models of fundamental ecological niches and species’ distributional areas. Biodiversity Informatics. 2005;2:1–10. [Google Scholar]
- 27.Peterson A, Soberón J, Pearson R, Anderson R, Martínez-Meyer E, Nakamura M, et al. Ecological niches and geographic distributions Princeton University Press; Princeton, NJ: 328pp. 2011. [Google Scholar]
- 28.Townsend Peterson A, Papeş M, Eaton M. Transferability and model evaluation in ecological niche modeling: a comparison of GARP and Maxent. Ecography. 2007;30(4):550–60. [Google Scholar]
- 29.Peterson AT, Papeş M, Soberón J. Rethinking receiver operating characteristic analysis applications in ecological niche modeling. PLoS ONE. 2008;213(1):63–72. [Google Scholar]
- 30.Owens HL, Campbell LP, Dornak LL, Saupe EE, Barve N, Soberón J, et al. Constraints on interpretation of ecological niche models by limited environmental ranges on calibration areas. Ecological Modelling. 2013;263:10–8. [Google Scholar]
- 31.Ruiz-Fons F, Fernández-de-Mera IG, Acevedo P, Gortázar C, de la Fuente J. Factors driving the abundance of Ixodes ricinus ticks and the prevalence of zoonotic I. ricinus-borne pathogens in natural foci. Applied and Environmental Microbiology. 2012;78(8):2669–76. doi: 10.1128/AEM.06564-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Daniel M, Danielová V, Kříž B, Kott I. An attempt to elucidate the increased incidence of tick-borne encephalitis and its spread to higher altitudes in the Czech Republic. International Journal of Medical Microbiology Supplements. 2004;293:55–62. [DOI] [PubMed] [Google Scholar]
- 33.Gray JS. Ixodes ricinus seasonal activity: implications of global warming indicated by revisiting tick and weather data. International Journal of Medical Microbiology. 2008;298:19–24. [Google Scholar]
- 34.Gage KL, Burkot TR, Eisen RJ, Hayes EB. Climate and vectorborne diseases. American Journal of Preventive Medicine. 2008;35(5):436–50. doi: 10.1016/j.amepre.2008.08.030 [DOI] [PubMed] [Google Scholar]
- 35.Gray J. The development and seasonal activity of the tick Ixodes ricinus: a vector of Lyme borreliosis. Review of Medical and Veterinary Entomology. 1991;79(6):323–33. [Google Scholar]
- 36.Lindgren E, Jaenson TG. Lyme borreliosis in Europe: influences of climate and climate change, epidemiology, ecology and adaptation measures. WHO Regional Office for Europe Copenhagen; 2006.
- 37.Jaenson TG, Jaenson DG, Eisen L, Petersson E, Lindgren E. Changes in the geographical distribution and abundance of the tick Ixodes ricinus during the past 30 years in Sweden. Parasites & Vectors. 2012;5(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Materna J, Daniel M, Danielová V. Altitudinal distribution limit of the tick Ixodes ricinus shifted considerably towards higher altitudes in central Europe: results of three years monitoring in the Krkonose Mts. (Czech Republic). Central European Journal of Public Health. 2005;13(1):24–8. [PubMed] [Google Scholar]
- 39.Zeman P, Pazdiora P, Benes C. Spatio-temporal variation of tick-borne encephalitis (TBE) incidence in the Czech Republic: is the current explanation of the disease’s rise satisfactory? Ticks and Tick-borne Diseases. 2010;1(3):129–40. doi: 10.1016/j.ttbdis.2010.05.003 [DOI] [PubMed] [Google Scholar]
- 40.Filippova NA. Forms of sympatry and possible ways of microevolution of closely related species of the group Ixodes ricinus–persulcatus (Ixodidae). Acta Zoologica Lituanica. 2002;12(3):215–27. [Google Scholar]
- 41.Zimmermann NE, Edwards TC, Graham CH, Pearman PB, Svenning JC. New trends in species distribution modelling. Ecography. 2010;33(6):985–9. [Google Scholar]
- 42.Araújo MB, Luoto M. The importance of biotic interactions for modelling species distributions under climate change. Global Ecology and Biogeography. 2007;16(6):743–53. [Google Scholar]
- 43.Paulauskas A, Galdikaitė-Brazienė E, Radzijevskaja J, Aleksandravičienė A, Galdikas M. Genetic diversity of Ixodes ricinus (Ixodida: Ixodidae) ticks in sympatric and allopatric zones in Baltic countries. Journal of Vector Ecology. 2016;41(2):244–53. doi: 10.1111/jvec.12219 [DOI] [PubMed] [Google Scholar]
- 44.Kovalev S, Golovljova I, Mukhacheva T. Natural hybridization between Ixodes ricinus and Ixodes persulcatus ticks evidenced by molecular genetics methods. Ticks and tick-borne diseases. 2016;7(1):113–8. doi: 10.1016/j.ttbdis.2015.09.005 [DOI] [PubMed] [Google Scholar]
- 45.Milne A. The ecology of the sheep tick, Ixodes ricinus L. Host relationships of the tick: Part 2. Observations on hill and moorland grazings in northern England. Parasitology. 1949;39(3–4):173–97. [DOI] [PubMed] [Google Scholar]
- 46.Hasle G. Transport of ixodid ticks and tick-borne pathogens by migratory birds. Frontiers in Cellular and Infection Microbiology. 2013;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jaenson TG, Lindgren E. The range of Ixodes ricinus and the risk of contracting Lyme borreliosis will increase northwards when the vegetation period becomes longer. Ticks and Tick-borne Diseases. 2011;2(1):44–9. doi: 10.1016/j.ttbdis.2010.10.006 [DOI] [PubMed] [Google Scholar]
- 48.Diuk-Wasser MA, Vannier E, Krause PJ. Coinfection by Ixodes tick-borne pathogens: ecological, epidemiological, and clinical consequences. Trends in Parasitology. 2016;32(1):30–42. doi: 10.1016/j.pt.2015.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
These records were drawn from the Global Biodiversity Information Facility (GBIF), VectorMap, and the scientific literature.
(CSV)
This supporting information describes the detailed results of model evaluation based on partial ROC tests applied to 5 random subsets of occurrence data.
(PDF)
The testing records are those retained from the original occurrences (i.e. black dots) but none coincided with the 416 records used in model calibration (i.e. yellow circles).
(PDF)
This supporting information shows the results regarding the relative contributions of six environmental variables to models for I. ricinus based on 5 random subsets of occurrences.
(PDF)
(CSV)
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files. GeoTIFF dataset for different general circulation models are openly available via Figshare repository (https://doi.org/10.6084/m9.figshare.5067373).