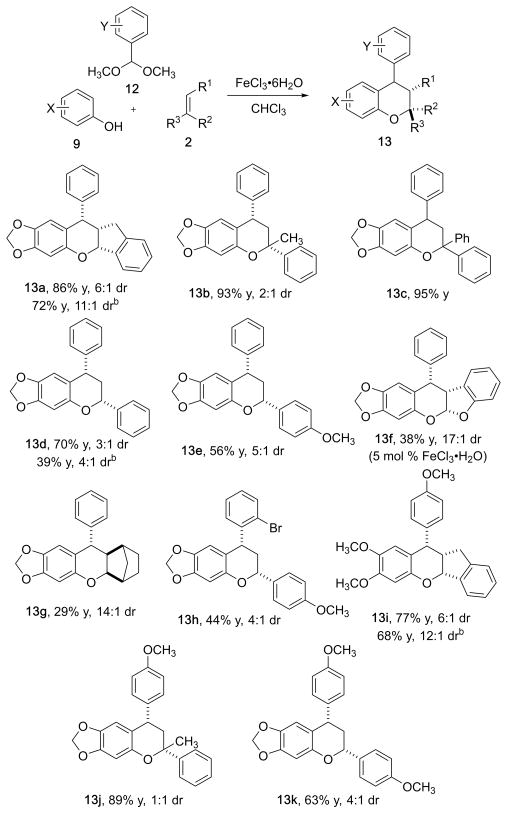
Figure 4.
Multicomponent condensation reaction with acetals, phenols, and olefins.a a Reaction conditions: 2.0 mmol of phenol, 2.0 mmol of acetal, and 1.0 mmol of dienophile, 0.5 M with respect to and 10 mol % FeCl3·6H2O with respect to phenol unless otherwise stated; isolated yields; diastereomeric ratio determined by 1H NMR; detailed experimental conditions are provided in the Supporting Information. b Yield and dr after trituration with hot hexanes.