Table 1.
Catalyzed Hetero-Diels–Alder of o-Quinone Methidesa
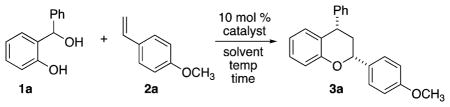
| |||||
|---|---|---|---|---|---|
| entry | catalyst | solvent | time (h) | yield (%)b | drc |
| 1 | MgCl2 | CH2Cl2 | 1 | <5 | — |
| 2 | Pd(TFA)2 | CH2Cl2 | 5 | 22 | 2:1 |
| 3 | FeCl3·6H2O | CH2Cl2 | 6 | 36 | 6:1 |
| 4 | FeCl3 | CH2Cl2 | 1 | 57 | 4:1 |
| 5d | FeCl3, 4 Å MS | CH2Cl2 | 1 | 28 | 1:1 |
| 6 | TMSCl/MeOH | CH2Cl2 | 1 | 26 | 4:1 |
| 7e | FeCl3 | CHCl3 | 1.3 | 82 | 4:1 |
Reaction conditions: diol (0.5 mmol, 1 equiv), p-methoxystyrene (2 equiv), catalyst (10 mol % with respect to diol) and 0.5 M with respect to diol, 0 °C to rt.
Isolated yields.
diastereomeric ratio determined by 1H NMR
4 Å MS (200 mg),
CHCl3 stabilized with amylenes.
