Fig. 1.
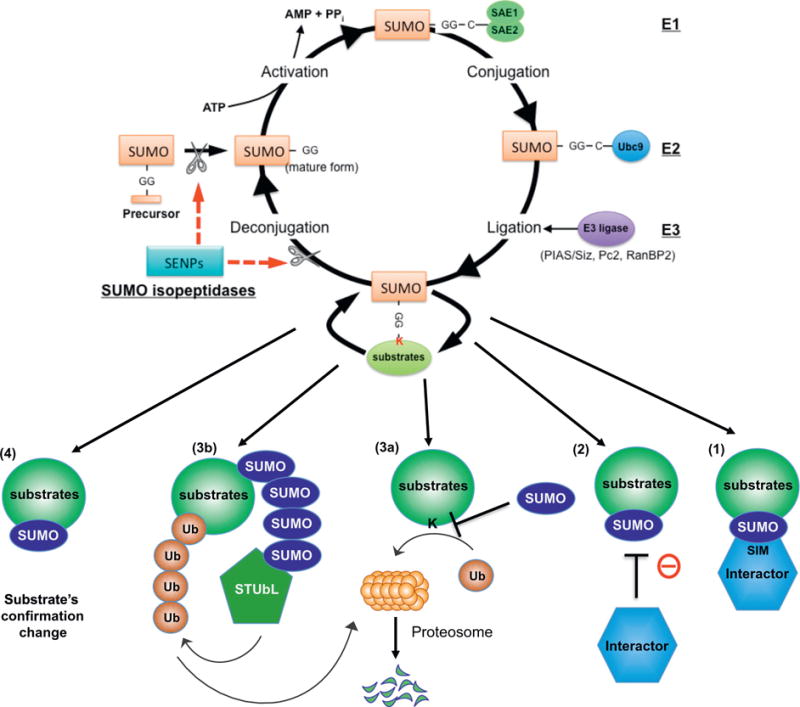
SUMOylation system and its regulatory mechanism. The SUMOylation system is a dynamic process of protein modification achieved by two enzyme systems; one which consisting of conjugates SUMO to substrates and one which de-conjugates. [176–179] SUMO proteins are covalently attached to certain residues of specific target substrates and change the function of these substrates. Prior to conjugation, the E1-activating enzyme, SAE1–SAE2 heterodimer, activates the mature form of SUMO [180]. SUMO is then transferred to Ubc9, an E2 conjugase, forming a thioester bond between Ubc9 and SUMO [181]. Lastly, SUMO E3 ligases, including a family of protein inhibitors such as activated STAT (PIAS1-4), transfer SUMO to the target substrate containing the free е-amino group of a lysine residue [182]. De-SUMOylation enzymes are also involved in the process of SUMOylation. Sentrin/SUMO-specific proteases (SENPs; SENP1–7) catalyze both de-conjugation of SUMOylated substrates and editing of the SUMO precursor into the matured form which now have a pair of glycine residues at the C terminus [183,184]. Adapted, reprinted, and modified from Heo et al. [22] with permission from ANTIOXIDANTS AND REDOX SIGNALING, April 2016, published by Mary Ann Liebert, Inc., New Rochelle, NY.
