Abstract
Natural herbal compounds have been widely introduced as an alternative therapeutic approach in cancer therapy. Despite potent anticancer activity of curcumin, its clinical application has been limited because of low water solubility and resulting poor bioavailability. In this study, we designed a novel ultrasonic-assisted method for the synthesis of curcumin-loaded chitosan–alginate–sodium tripolyphosphate nanoparticles (CS-ALG-STPP NPs). Furthermore, antitumor effect of curcumin-loaded NPs was evaluated in vitro. Field emission scanning electron microscopy (FE-SEM) and atomic force microscopy (AFM) were used to characterize the properties of NPs. Antitumor activity of curcumin-loaded NPs was assessed by using MTT and quantitative real-time polymerase chain reaction (qRT-PCR). FE-SEM and AFM data revealed the spherical morphology, and the average size of NPs was <50 nm. In vitro cytotoxicity assay suggested that curcumin-loaded CS-ALG-STPP NPs displayed significant antitumor activity compared with the free curcumin. Gene expression level analyses showed that curcumin NPs significantly increased the apoptotic gene expression. Collectively, our results suggest that curcumin-loaded NPs significantly suppressed proliferation and promoted the induction of apoptosis in human cervical epithelioid carcinoma cancer cells, which might be regarded as an effective alternative strategy for cancer therapy.
Keywords: cancer, curcumin, biodegradable nanoparticles, antitumor activity, apoptosis induction
Introduction
Curcumin is a yellow polyphenol derived from the turmeric rhizome (Curcuma longa).1 A growing number of studies have indicated that curcumin has several beneficial properties such as antitumor,2,3 antioxidant,4 anti-amyloid,5 anti-inflammatory,6 antimicrobial,7 and wound healing effects.8 Previous studies suggested that curcumin alone or in combination with other anticancer drugs possesses potent antitumor activity on different tumor cell lines including hepatic,9 prostatic,10 ovarian,11 breast,12 pancreatic,13 and gastric carcinomas.14 Furthermore, it has been reported that curcumin has significant effects on carcinogenesis signaling pathways through angiogenesis inhibition,13 cell death activation,15 and cell cycle arrest induction.16 Despite promising antitumor activity of curcumin, its clinical application has been hampered due to rapid systemic elimination and low aqueous solubility that limits the bioavailability of this bioactive compound.17,18 To overcome these limitations, different approaches have been used to improve curcumin solubility including the encapsulation of curcumin in liposome,19 dendrimers,20 polymeric nanoparticles (NPs),21 nanogel,22 cyclodextrin,23 and biodegradable microsphere.24 Because of the hydrophobic nature of curcumin, its encapsulation by natural biocompatible and biodegradable polymers has been introduced as a promising strategy for cancer therapy. Alginate (ALG) and chitosan (CS) are two natural biopolymers that have numerous pharmaceutical and biomedical applications.25 ALG is a water-soluble linear polysaccharide extracted from brown seaweed, and ALG NPs can be obtained by adding sodium tripolyphosphate (STPP) and polycationic solution, resulting in the formation of polyelectrolyte complex.26 Because of low immunogenicity and nontoxic properties of CS, it has been selected as an alternative cationic polymer.27 Ample evidence has shown that curcumin NPs can be prepared by using CS28,29 or ALG.30,31 In addition, a previous study suggested that curcumin can be encapsulated with ALG, CS, and pluronic composite, which could significantly enhance the anticancer activity of curcumin.32 Furthermore, curcumin has been incorporated into CS-ALG sponge to improve its wound healing property.33,34 More recently, curcumin NPs have been produced by using CS, ALG, TPP, and calcium chloride.35 Regarding the necessity of finding an alternative drug delivery system for cancer therapy, the present study describes a new approach for loading curcumin onto CS, ALG, and STPP NPs by an ultrasonic-assisted method. Then, the effects of curcumin-loaded NPs on proliferation and apoptosis were investigated in human cervical epithelioid carcinoma (HeLa) cancer cell lines.
Materials and methods
Chemicals
Curcumin powder was purchased from Merck Company, Darmstadt, Germany. CS and ALG were obtained from Sigma-Aldrich (St Louis, MO, USA). STPP was prepared from Daejung, Sasang-gu, Busan, South Korea. Culture medium materials and MTT were purchased from ATOCEL (Graz, Austria) and Alfa Aesar (Ward Hill, MA, USA), respectively.
Preparation of curcumin-loaded CS-ALG-STPP NPs
CS-ALG-STPP NPs were prepared according to Caetano et al’s report, with some modifications.36 Ten milligram of CS was dissolved in 1% acetic acid to reach a final concentration of 1 mg/mL by magnetic stirring, and its pH was adjusted to 4.9. CS solution was stirred by ultrasonic irradiation (Bandelin, Berlin, Germany) for 1 hour. Curcumin powder was then dissolved in a small quantity of ethanol, and 1 mL of its solution (1 mg/mL in ethanol) was added in CS solution with stirring; 5 mg of ALG was dissolved in distilled water, and pH of the suspension was adjusted to 4.6 by HCl (0.5 M). ALG was added dropwise to this solution, followed by drop-wise addition of STPP (0.13% w/v in ultrapure deionized water) using an insulin syringe (flow rate: 1 mL/min) under high-speed stirring for 120 minutes. The resulting mixture was collected and washed with 10% ethanol and water by centrifugation at 14,000 rpm for 30 minutes at 4°C.
NP characterization
The surface morphology and size distribution of curcumin-loaded ALG-CS-STPP NPs were characterized by field emission scanning electron microscopy (FE-SEM; Mira 3 XMU; TESCAN, Brno, Czech Republic), atomic force microscopy (AFM; Easyscan 2 Flex, Liestal, Switzerland), and Fourier transform infrared (FT-IR) spectroscopy. The FT-IR spectroscopy of curcumin, ALG-CS-STPP NPs, and curcumin-loaded NPs were obtained by using FT-IR spectrophotometer (Vector 22 FT-IR; Bruker Optik GmbH, Ettlingen, Germany).
Encapsulation efficiency
To determine the amount of curcumin entrapment, high-performance liquid chromatography (HPLC) analysis (Knauer; Smartline, Berlin, Germany) was performed. As we mentioned previously, at the final step of NP preparation, curcumin–CS-ALG-STPP solution was centrifuged, and the decanted solution was collected. After washing the mixture with ethanol, supernatant was collected, and the amount of remaining curcumin was evaluated. In addition, 1 mg of air-dried NPs was dissolved in 1 mL of water, and curcumin loaded onto NPs has been extracted using acetonitrile. The HPLC column was C18 (Eurospher, Knauer; Smartline, Berlin, Germany), and the mobile phase was a mixture of acetonitrile and water in a ratio of 90:10 (v/v). The flow rate of the mobile phase was 1 mL/min, and the eluent was detected by ultraviolet (UV) detector at a wave length of 425 nm.
Area under the peaks was measured by using EZChrom software, and then, the amount of curcumin was calculated using a standard curve. Curcumin loading efficiency was calculated using the following formula:37
In vitro release studies
The in vitro release of curcumin from NPs was carried out according to a previous report.32 Briefly, 5 mg of air-dried curcumin-loaded NPs was dispersed in 32 mL PBS (pH 7.4), and the resulting solution was divided and kept in 16 microfuge tubes (each 2 mL). At time intervals of 0, 1, 2, 3, 4, 5, and 24 hours, the solution was centrifuged at 10,000 rpm for 10 minutes. The released curcumin was dissolved in 2 mL absolute ethanol, and the absorbance was measured using UV spectrophotometer (SQ4802; UNICO, Dayton, NJ, USA) at a wavelength of 425 nm. The in vitro release profile was measured by using the following equation:
where Ct represents the concentration of released curcumin at the time t, and C0 is the total amount of curcumin loaded in CS-ALG-STPP NPs.
Adsorption isotherm study
Isotherm experiments were performed according to a previous report by Cheng et al.38 In brief, different concentrations of curcumin (0.1, 0.15, 0.2, 0.25, and 0.3 mg/mL) were mixed with CS-ALG-STPP NPs, and the resulting mixture was continuously shaken for 24 hours at 25°C. The adsorption capacity of CS-ALG-STPP NPs for curcumin was calculated by using the following equation:
where C0 is considered as the initial concentration of curcumin (mg/mL), and Ce is the curcumin concentration in equilibrium state; V and m are the total volume of the mixture (mL) and the total mass of CS-ALG-STPP NPs, respectively; and qe and Ce values (as dependent variables) were obtained through the experiments. In order to determine the curcumin adsorption onto CS-ALG-STPP NPs and the maximum uptake (qmax), data were fitted to several isotherm equations including Langmuir, Freundlich, Temkin, Elovich, and Langmuir–Freundlich.
Cell culture assays
To evaluate the cellular uptake of curcumin, HeLa cancer cell line was purchased from Pasture Institute, Tehran, Iran. Cells were cultured in Roswell Park Memorial Institute medium supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin at 37°C in CO2 incubator. The cells were treated with 50 µg/mL free curcumin or curcumin-loaded NPs. After 24 hours, the treated cells were washed with PBS and then examined under an inverted fluorescence microscope (Nikon Instruments, Melville, NY, USA). In order to assess the cytotoxic effects of curcumin-loaded NPs, human foreskin fibroblasts and HeLa cells with a density of 7×103 were seeded in 96-well plates and incubated with different concentrations of free and encapsulated curcumin for 48 hours. Then, MTT (5 mg/mL) was added to each well, and the cells were incubated for 4 hours at 37°C. For dissolving the blue formazan precipitate, dimethyl sulfoxide was added, and the absorbance was determined at 570 nm by an ELISA reader (BioTek, Winooski, VT, USA). The percentage of cell viability was obtained by using the following equation:
All cell culture assessments were performed in triplicate.
Nuclear staining
Like MTT assay, cells from the HeLa cell line were cultured and incubated with blank NPs, curcumin, or curcumin-loaded NPs for 48 hours. Then, the cells were washed with PBS and fixed with cold 4% paraformaldehyde for 20 minutes. After washing with PBS, for enhancing the permeability, 0.2% Triton X-100 was added for 20 minutes; at the final stage, nuclear staining was performed using DAPI (1 µg/mL; Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA) for 25 minutes, and the cells were observed under a fluorescence microscope (Nikon).
Quantitative real-time polymerase chain reaction (qRT-PCR)
HeLa cells were incubated with blank NPs, curcumin, and curcumin-loaded CS-ALG-STPP NPs (50 µg/mL) for 48 hours. Then, they were washed with PBS, and RNA was extracted using total RNA extraction kit (Yekta Tajhiz, Tehran, Iran) according to the manufacturer’s protocol. cDNA synthesis was performed by a commercially available reverse transcriptase kit (Yekta Tajhiz). A total 20 µL of reaction mixture contained 0.4 µL forward primer, 0.4 µL reverse primer, 2 µL cDNA, 10 µL SYBR Green PCR master mix (Yekta Tajhiz), 0.4 µL carboxy-X-rhodamine (Yekta Tajhiz), and 6.8 µL RNase-free water. GAPDH was used as an internal control. Gene expression level was analyzed by Ct method. Table S1 shows the primer sequences and their annealing temperature.
Statistical analysis
MTT assay data were analyzed using unpaired Student’s t-test. The qRT-PCR results were analyzed by using one-way analysis of variance, followed by Tukey’s post hoc test. The results are expressed as mean ± standard error of mean, and p-values ≤0.05 were considered statistically significant.
Results
Curcumin-loaded CS-ALG-STPP NP properties
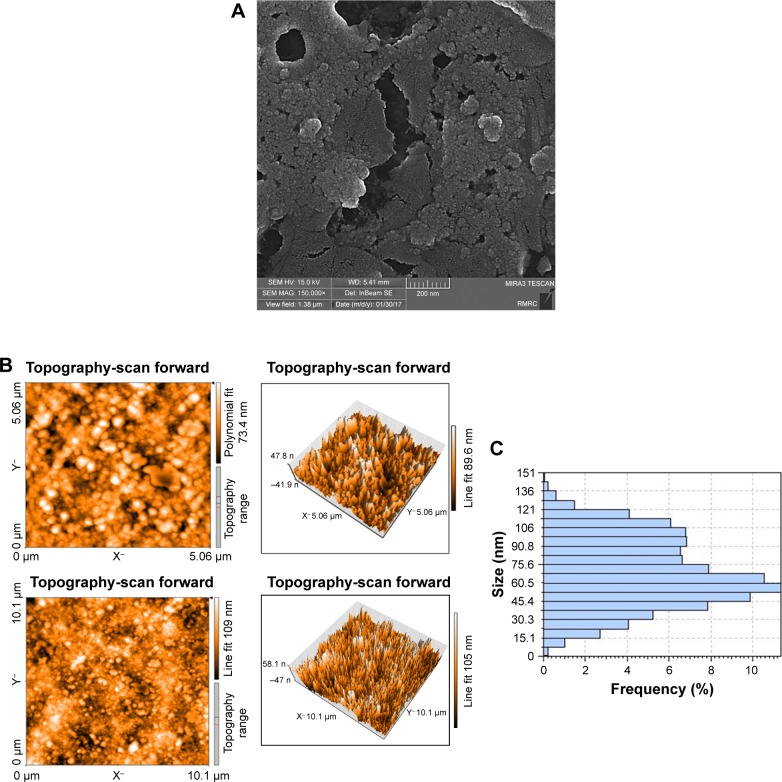
FE-SEM results showed that curcumin-loaded CS-ALG-STPP NPs were spherical, with a mean size of ~50 nm, which are agglomerated together (Figure 1A). Figure 1B displays an AFM image and its three-dimensional view. Consistent with FE-SEM data, the quantification of AFM results also indicated that particles have uniform size distribution, and the average size of curcumin-loaded NPs was 50 nm (Figure 1C).
Figure 1.
Curcumin-loaded CS-ALG-STPP NPs characterization.
Notes: (A) FE-SEM data (magnification: 150,000×). (B) Two and three dimensional images of AFM result. (C) Quantification of AFM data indicated that curcumin-loaded NPs had spherical shape with an average size of 50 nm.
Abbreviations: AFM, atomic force microscopy; CS-ALG-STPP NPs, chitosan–alginate–sodium tripolyphosphate nanoparticles; FE-SEM, field emission scanning electron microscopy.
FT-IR spectroscopy analysis
Figure 2 shows the FT-IR spectra of curcumin, CS-ALG-STPP, and curcumin-loaded CS-ALG-STPP NPs. In the spectrum of CS-ALG-STPP NPs, the broad band at 3,432 cm−1 corresponds to hydroxyl and amine groups. The peaks near 1,630 and 1,413 cm−1 are attributed to the symmetric and asymmetric stretching vibrations of COO− groups, respectively. Moreover, the band around 1,026 cm−1 belongs to the amide bands. The peak at 1,087 cm−1 is due to the presence of –CH–OH in cyclic alcohol and C–O stretch. For curcumin, the peak at 3,478 cm−1 corresponds to amine groups. The adsorption peak at 1,510 cm−1 is observed, which corresponds to the C=O and C=C vibrations of curcumin. In addition, an absorption peak for C=C double bands at 1,627 cm−1 is not clear in this spectrum because of its overlapping with CS-ALG-STPP peak. The relative peak intensities of curcumin-loaded CS-ALG-STPP NPs are slightly weak, which may be due to the attachment of curcumin on functional groups of CS-ALG matrix.
Figure 2.
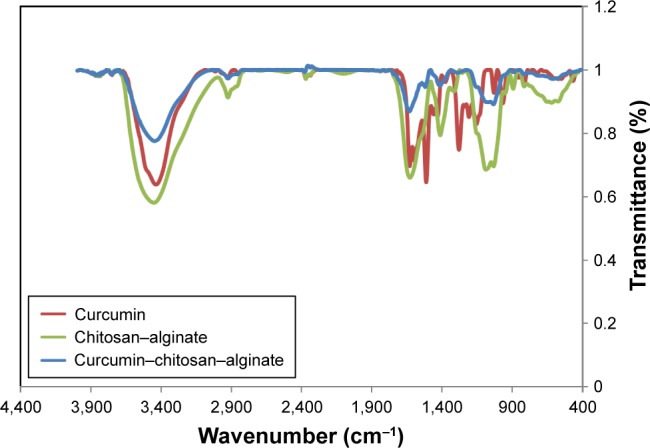
FT-IR spectra of curcumin, CS-ALG, and curcumin-loaded CS-ALG-STPP.
Abbreviations: CS-ALG-STPP, chitosan–alginate–sodium tripolyphosphate; FT-IR, Fourier transform infrared.
Entrapment efficiency
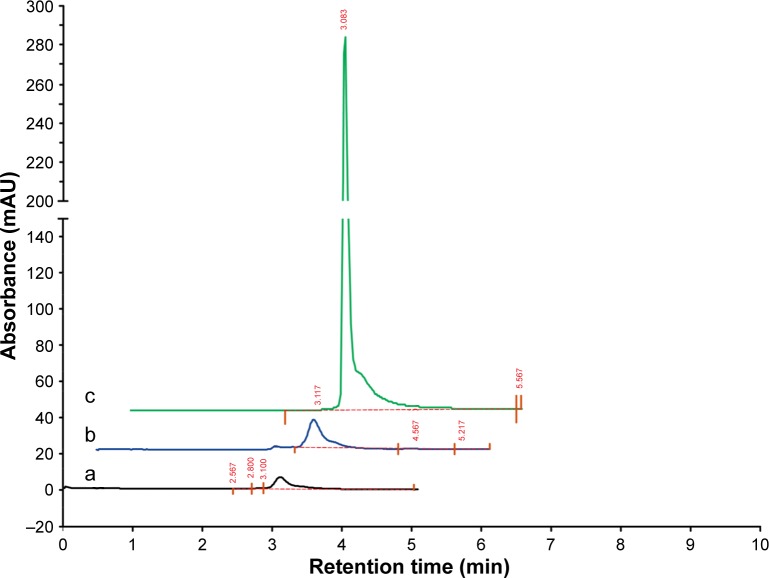
In order to assess the encapsulation efficiency, HPLC analysis was performed on decanted solution, solution after washing with ethanol, and curcumin-loaded CS-ALG-STPP NPs mixture in water. By calculating the area under the peaks, the entrapment efficiency for NP preparation was about 70% (Figure 3).
Figure 3.
Entrapment efficiency.
Notes: HPLC analysis for (a) decanted solution, (b) solution after washing with ethanol, and (c) curcumin-loaded CS-ALG-STPP NPs in aqueous solution.
Abbreviations: CS-ALG-STPP NPs, chitosan–alginate–sodium tripolyphosphate nanoparticles; HPLC, high-performance liquid chromatography.
In vitro release of curcumin from CS-ALG-STPP NPs
The in vitro drug release profile was evaluated at a predetermined time, and the percentage of curcumin released from CS-ALG-STPP NPs was measured by using a standard curve. Figure 4 shows the in vitro drug release profile of curcumin-loaded NPs. Our data indicated that 24% and 29% of curcumin were released from NPs after 1 and 2 hours, respectively. Then, a highly controlled release was observed till 24 hours, and 53% of physically loaded curcumin was released during this period (Figure 4).
Figure 4.
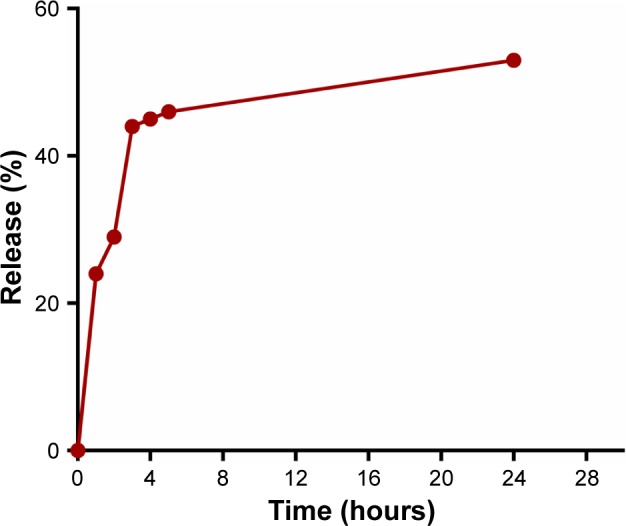
In vitro release kinetics of curcumin-loaded CS-ALG-STPP NPs.
Abbreviation: CS-ALG-STPP NPs, chitosan–alginate–sodium tripolyphosphate nano particle.
Adsorption affinity of curcumin and CS-ALG-STPP NPs
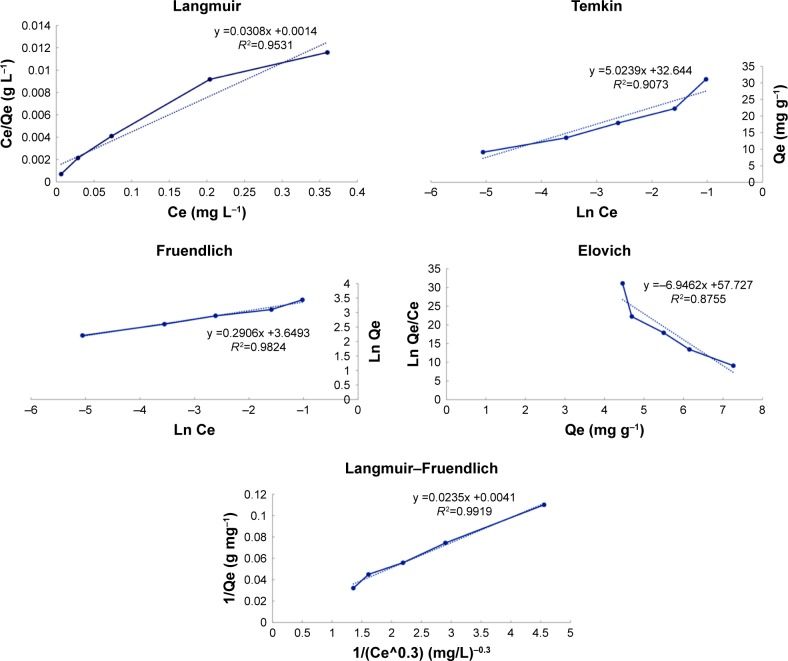
Isotherm process was carried out to assess the curcumin loading capacity onto CS-ALG-STPP NPs. Figure 5 depicts the adsorption isotherms for curcumin and CS-ALG-STPP NPs. Experimental results were fit to Langmuir, Freundlich, Temkin, Elovich, and Langmuir–Freundlich isotherm equations. Table 1 presents the adsorption isotherm data for curcumin on CS-ALG-STPP NPs and the equilibrium parameters. Regression analysis showed that among the mentioned isotherms, Langmuir–Freundlich isotherm model exhibits the highest linearity (R2=992).
Figure 5.
Adsorption isotherms of curcumin-loaded CS-ALG-STPP nanoparticles. Adsorption of curcumin onto CS-ALG-STPP NPs has been evaluated with different isotherm models, and regression analysis indicated that high linearity will be obtained using Langmuir–Freundlich isotherm model fit.
Abbreviation: CS-ALG-STPP NPs, chitosan–alginate–sodium tripolyphosphate nanoparticles.
Table 1.
Isotherm constants for adsorption of curcumin onto CS-ALG-STPP NPs
| Model | Parameters | Values |
|---|---|---|
| Langmuir isotherm | qm (mg g−1) | 32.467 |
| KL (L mg) | 22.000 | |
| R2 | 0.953 | |
|
| ||
| Freundlich isotherm | n | 3.441 |
| KF (mg1−(1/n) L1/n g−1) | 38.448 | |
| R2 | 0.982 | |
|
| ||
| Temkin isotherm | BT (J mol−1) | 5.024 |
| KT (L g−1) | 663.555 | |
| R2 | 0.907 | |
|
| ||
| Elovich isotherm | qm (mg g−1) | 0.144 |
| KE (L g−1) | 8.169*1025 | |
| R2 | 0.875 | |
|
| ||
| Langmuir–Freundlich | qm (mg g−1) | 243.902 |
| Kc | 0.174 | |
| n | 3.333 | |
| R2 | 0.992 | |
Abbreviations: CS-ALG-STPP NPs, chitosan–alginate–sodium tripolyphosphate nanoparticles; qm, maximum curcumin uptake.
The maximum curcumin uptake (qm) was obtained; 243.902 mg of curcumin was adsorbed per gram of CS-ALG-STPP NPs. According to this model, intermolecular interactions have an important role in the adsorption of curcumin onto CS-ALG-STPP NPs.
In vitro assays
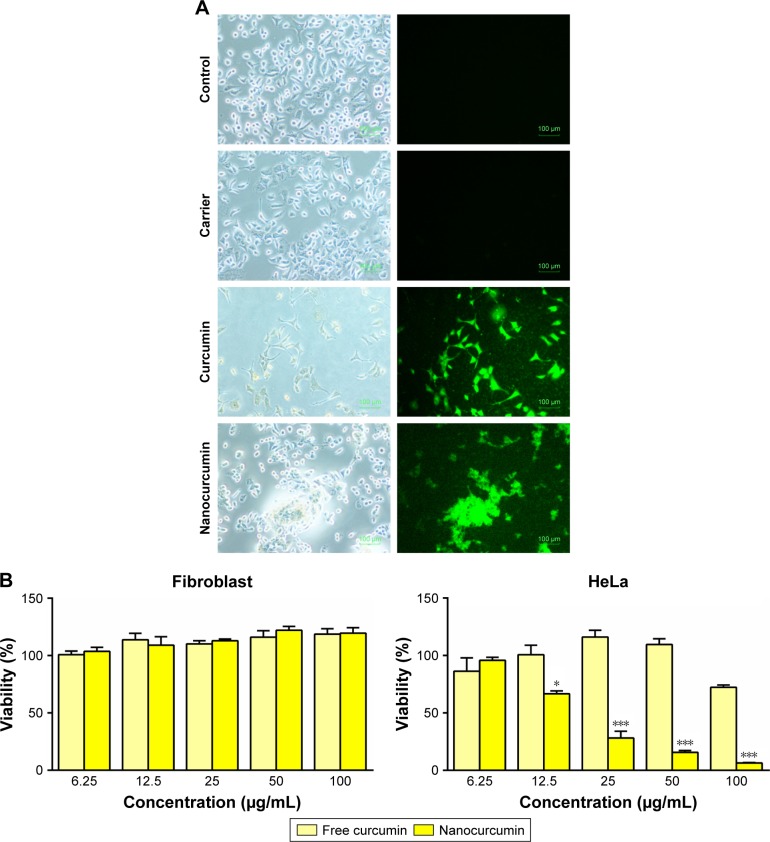
Based on the intrinsic fluorescence activity of curcumin, its uptake was evaluated in HeLa cells, 48 hours after curcumin-loaded CS-ALG-STPP NP incubation. Florescence microscopic images indicated that control cells and those incubated with CS-ALG-STPP NPs as blank NPs experimental group did not show any fluorescence activity. In contrast to control and blank NPs (carrier) experimental groups, HeLa cells treated with free curcumin or curcumin NPs (50 µg/mL) robustly exhibited green fluorescence (Figure 6A). In the next step, the cytotoxic effect of curcumin-loaded NPs was assessed by using MTT assay. Human foreskin fibroblasts and HeLa cells were incubated by different doses of free curcumin or curcumin-loaded CS-ALG-STPP NPs (6.25, 12.5, 25, 50, and 100 µg/mL) for 48 hours. To evaluate the toxicity of the curcumin NPs on normal cells, human foreskin fibroblasts were cultured and incubated with different concentrations of curcumin or curcumin NPs for 48 hours. The MTT assay results showed that significant cytotoxic effect was not detected in normal cultured cells under the treatment of curcumin or curcumin-loaded NPs (Figure 6B). Furthermore, the cytotoxic effect of curcumin-loaded NPs was assessed in HeLa cancer cell lines. Cell viability assay results showed that curcumin-loaded NPs, especially its high dose, had a significant dose-dependent inhibitory effect on cell proliferation compared with the free curcumin group (Figure 6B).
Figure 6.
In vitro assays.
Notes: (A) Bright field and fluorescence images of free curcumin and curcumin-loaded nanoparticles (50 µg/mL) uptake by HeLa cells. Scale bar: 100 µm; magnification 100×. (B) MTT results showed that curcumin-loaded nanoparticles have suppressed the proliferation of HeLa cells in a dose-dependent manner compared with free curcumin. *p<0.05 and ***p<0.001, n=3
Abbreviation: HeLa, human cervical epithelioid carcinoma.
Effect of curcumin-loaded NPs on apoptosis-related gene expression
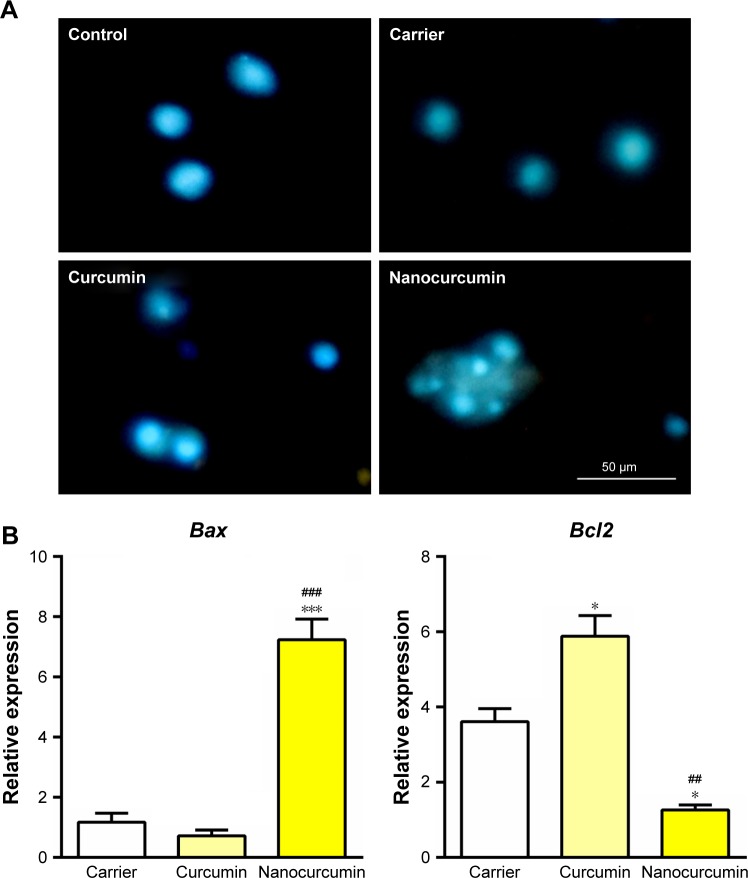
To assess the effect of curcumin-loaded CS-ALG-STPP NPs on apoptosis, HeLa cells were incubated with blank NPs, free curcumin, or curcumin-loaded CS-ALG-STPP NPs (50 µg/mL) for 48 hours. Nuclear staining was carried out using DAPI, and nucleolus changes were observed under fluorescent microscope. Our data showed that untreated and blank NPs receiving cells were stained equably blue fluorescence. In cells treated with curcumin-loaded CS-ALG-STPP NPs, DNA fragmentation, nucleolus pyknosis, and bright fluorescence emission, as apoptotic features, were observed. In contrast to curcumin-loaded NPs, curcumin treatment could not induce remarkable changes on nuclear morphology (Figure 7A).
Figure 7.
Effect of curcumin-loaded nanoparticles on cell apoptosis.
Notes: (A) Nuclear staining using DAPI showed that nucleolus morphological changes have been occurred in cells under the treatment of curcumin-loaded CS-ALG-STPP nanoparticles. Scale bar: 50 µm; magnification 200× (B) qRT-PCR data indicated that curcumin-loaded CS-ALG-STPP NPs significantly increased the apoptotic gene expression (Bax) and decreased the antiapoptotic gene expression (Bcl2) compared with carrier and free curcumin experimental groups. *p<0.05 and ***p<0.001 nanocurcumin compared with carrier group; ##p≤0.01 and ###p≤0.001 curcumin nanoparticles compared with free curcumin.
Abbreviations: CS-ALG-STPP NPs, chitosan–alginate–sodium tripolyphosphate nanoparticles; qRT-PCR, quantitative real-time polymerase chain reaction.
In order to determine the effect of curcumin-loaded CS-ALG-STPP NPs on apoptotic gene expression, qRT-PCR was performed. qRT-PCR data demonstrated that curcumin-loaded CS-ALG-STPP NPs significantly increased the level of Bax gene expression as apoptotic gene marker compared with the carrier and free curcumin groups. Curcumin-loaded NPs remarkably decreased the Bcl2 gene expression as antiapoptotic gene marker. In addition, the Bcl2 gene expression level was also significantly decreased in cells incubated with carrier compared with the free curcumin group (Figure 7B).
Discussion
Ample evidence has suggested that curcumin exerts a potent anticancer activity on different cancerous cells.39 However, because of poor aqueous solubility and low bioavailability, its clinical application has been limited.40 Development of novel formulation for the encapsulation of curcumin attracts a strong interest in nanomedicine field. An ideal NP should be stable in the long term, and for circulation in smallest capillary, their size should be <100 nm.38 In addition, all materials for NP synthesis should be biocompatible.38 Another important factor in NP manufacturing is the ability of particles for passing through blood-brain barrier.38 As mentioned previously, the size of NPs is an important parameter in successful internalization of particles to cancer cells.38 Several studies indicated that particle size in the range of 10–100 nm is ideal for cancer therapy.41 In our study, FE-SEM and AFM data showed that the size of curcumin-loaded CS-ALG-STPP NPs is <50 nm. A previous study by Das et al showed that curcumin-loaded NPs could be synthesized by applying ALG as the cross-linker into CS solution, and the size of the prepared particles was 100 nm.32 Another study showed that the size of curcumin-loaded CS NPs was >200 nm.42 In addition, Mirnejad et al used CS and TPP for curcumin loading, and particle size was 160 nm.29 In contrast to the mentioned studies, we could prepare curcumin-loaded NPs with smaller size. Probably, applying different concentrations of CS, ALG, and STPP and subsequent ultrasonication of final solution can result in the synthesis of NPs with desirable size in cancer therapeutic approach. In agreement with previous reports,29,32 our in vitro drug release results also showed 53% release of curcumin from NPs within 24 hours. Because of the nature of CS and its better solubility in acidic pH, the slower release of curcumin is occurred at a pH of 7.4.29 The initial burst release is perhaps due to the attachment of curcumin on the surface of NPs.43 After the initial burst, a sustained release was observed for curcumin-loaded NPs. The controlled release of drugs from NPs is considered as an essential factor in drug delivery approach.43
The encapsulation efficiency for curcumin-loaded CS-ALG-STPP NPs was obtained, which was about 70%. Mirnejad et al also showed that the encapsulation efficiency of curcumin-loaded CS-TPP NPs was 75%.29 However, Das et al showed that the entrapment efficiency of curcumin-loaded CS-ALG NPs is <20%.32 However, in the present study, we achieved a higher curcumin loading efficiency, which may result from the use of STPP as a cross-linker. Beside the NP size, the binding affinity between curcumin and CS-ALG-STPP NPs is regarded as a critical factor in NP manufacturing. Studying the adsorption process using different isotherm models showed that curcumin preferentially adsorbs to CS-ALG-STPP NPs by intermolecular interaction (Langmuir–Freundlich model). Previous studies have shown that curcumin can be adhered to amine-functionalized mesoporous silica (AAS-MS) NPs using intermolecular interaction of curcumin as well as its interaction with NPs.44 Due to the hydrophobic structure of curcumin and AAS-MS NPs, hydrophobic attractive forces are regarded as an important reason for intermolecular interactions.44 In the next step, the antitumor activity of curcumin-loaded CS-ALG-STPP NPs was investigated. MTT assay and qRT-PCR data suggested that, in contrast to free curcumin, curcumin-loaded NPs effectively inhibit HeLa cell proliferation and induce apoptosis, respectively. Previous reports have suggested that curcumin inhibits cancer cell proliferation and induces cell death,45 but in our study, free curcumin could not exert a significant antitumor activity compared with the curcumin-loaded NPs. This observation may result from very fine dispersion ability of curcumin-loaded CS-ALG-STPP NPs in aqueous solution and the possibility that this property might have been preserved for several months. Unlike our study, Das et al reported that curcumin-loaded CS-ALG NPs exhibit very poor solubility in aqueous solution, and therefore, pluronic F127 composite was used for enhancing the solubility of these NPs.32 The well-dispersion property and also the small size of NPs may lead to better anticancer activity of curcumin-loaded CS-ALG-STPP NPs compared with the free curcumin. Similar to the group received curcumin-loaded CS-ALG-STPP NPs, we have observed that the level of Bcl2 gene expression (as the antiapoptotic factor) has reduced in cells received blank NPs compared with the free curcumin. Previous studies have also demonstrated that CS possesses anticancer activity against several cancer cell lines including oral cancer cells.46 It has been shown that the cytotoxic effect of CS is mediated through the induction of apoptosis in oral cancer cells.47 Based on such evidence, the significant reduction in Bcl2 gene expression may result from the anticancer property of CS. However, we did not find any significant difference on Bax gene expression between groups treated by free curcumin or blank NPs.
Conclusion
In conclusion, curcumin-loaded NPs were successfully prepared by using ultrasonication of biodegradable polymers including CS and ALG. Curcumin-loaded CS-ALG-STPP NPs were homogenously dispersed in aqueous solution, and their ideal size for cancer therapy was obtained. These NPs could be internalized into HeLa cancer cells, and the incubation of HeLa cells with curcumin-loaded CS-ALG-STPP NPs led to suppression of proliferation. In addition, the expression of apoptotic genes was increased in cells treated by curcumin-loaded NPs. These findings may provide a novel therapeutic approach in cancer therapy.
Supplementary material
Table S1.
Primer sequences
| Primer name | Sequence (5′→3′) | Size (bp) | Annealing temperature (°C) |
|---|---|---|---|
| GAPDH | Forward: GGTGGTCTCCTCTGACTTCAACA; reverse: GTTGCTGTAGCAAATTCGTTGT | 126 | 60 |
| Bax | Forward: TGG CAG CTG ACA TGT TTT CTG AC; reverse: TCA CCC AAC CAC CCT GGT CTT | 195 | 60 |
| Bcl2 | Forward: TCG CCC TGT GGATGA CTG A; reverse: CAGAGA CAG CCA GGA GAA ATC A | 134 | 60 |
Acknowledgments
This work was supported by a grant (Number 9503023) from Student Research Committee, Babol University of Medical Sciences, Babol, Mazandaran, Iran. The authors are thankful to Dr Alireza Mani for editing the article.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Cao J, Jiang LP, Liu Y, Yang G, Yao XF, Zhong LF. Curcumin-induced genotoxicity and antigenotoxicity in HepG2 cells. Toxicon. 2007;49(8):1219–1222. doi: 10.1016/j.toxicon.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 2.Sa G, Das T. Anti cancer effects of curcumin: cycle of life and death. Cell Div. 2008;3(1):14. doi: 10.1186/1747-1028-3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Salem M, Rohani S, Gillies ER. Curcumin, a promising anti-cancer therapeutic: a review of its chemical properties, bioactivity and approaches to cancer cell delivery. RSC Adv. 2014;4(21):10815–10829. [Google Scholar]
- 4.Motterlini R, Foresti R, Bassi R, Green CJ. Curcumin, an antioxidant and anti-inflammatory agent, induces heme oxygenase-1 and protects endothelial cells against oxidative stress. Free Radic Biol and Med. 2000;28(8):1303–1312. doi: 10.1016/s0891-5849(00)00294-x. [DOI] [PubMed] [Google Scholar]
- 5.Ringman JM, Frautschy SA, Cole GM, Masterman DL, Cummings JL. A potential role of the curry spice curcumin in Alzheimer’s disease. Curr Alzheimer Res. 2005;2(2):131–136. doi: 10.2174/1567205053585882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jurenka JS. Anti-inflammatory properties of curcumin, a major constituent of Curcuma longa: a review of preclinical and clinical research. Altern Med Rev. 2009;14(2):141–153. [PubMed] [Google Scholar]
- 7.De R, Kundu P, Swarnakar S, et al. Antimicrobial activity of curcumin against Helicobacter pylori isolates from India and during infections in mice. Antimicrob Agents Chemother. 2009;53(4):1592–1597. doi: 10.1128/AAC.01242-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Phan TT, See P, Lee ST, Chan SY. Protective effects of curcumin against oxidative damage on skin cells in vitro: its implication for wound healing. J Trauma. 2001;51(5):927–931. doi: 10.1097/00005373-200111000-00017. [DOI] [PubMed] [Google Scholar]
- 9.Notarbartolo M, Poma P, Perri D, Dusonchet L, Cervello M, D’Alessandro N. Antitumor effects of curcumin, alone or in combination with cisplatin or doxorubicin, on human hepatic cancer cells. Analysis of their possible relationship to changes in NF-kB activation levels and in IAP gene expression. Cancer Lett. 2005;224(1):53–65. doi: 10.1016/j.canlet.2004.10.051. [DOI] [PubMed] [Google Scholar]
- 10.Dorai T, Cao YC, Dorai B, Buttyan R, Katz AE. Therapeutic potential of curcumin in human prostate cancer. III. Curcumin inhibits proliferation, induces apoptosis, and inhibits angiogenesis of LNCaP prostate cancer cells in vivo. Prostate. 2001;47(4):293–303. doi: 10.1002/pros.1074. [DOI] [PubMed] [Google Scholar]
- 11.Lin YG, Kunnumakkara AB, Nair A, et al. Curcumin inhibits tumor growth and angiogenesis in ovarian carcinoma by targeting the nuclear factor-κB pathway. Clin Cancer Res. 2007;13(11):3423–3430. doi: 10.1158/1078-0432.CCR-06-3072. [DOI] [PubMed] [Google Scholar]
- 12.Ramachandran C, Rodriguez S, Ramachandran R, et al. Expression profiles of apoptotic genes induced by curcumin in human breast cancer and mammary epithelial cell lines. Anticancer Res. 2005;25(5):3293–3302. [PubMed] [Google Scholar]
- 13.Kunnumakkara AB, Guha S, Krishnan S, Diagaradjane P, Gelovani J, Aggarwal BB. Curcumin potentiates antitumor activity of gemcitabine in an orthotopic model of pancreatic cancer through suppression of proliferation, angiogenesis, and inhibition of nuclear factor-κB–regulated gene products. Cancer Res. 2007;67(8):3853–3861. doi: 10.1158/0008-5472.CAN-06-4257. [DOI] [PubMed] [Google Scholar]
- 14.Cai XZ, Wang J, Li XD, et al. Curcumin suppresses proliferation and invasion in human gastric cancer cells by down-regulation of PAK1 activity and cyclin D1 expression. Cancer Biol Ther. 2009;8(14):1360–1368. doi: 10.4161/cbt.8.14.8720. [DOI] [PubMed] [Google Scholar]
- 15.Reuter S, Eifes S, Dicato M, Aggarwal BB, Diederich M. Modulation of anti-apoptotic and survival pathways by curcumin as a strategy to induce apoptosis in cancer cells. Biochem Pharmacol. 2008;76(11):1340–1351. doi: 10.1016/j.bcp.2008.07.031. [DOI] [PubMed] [Google Scholar]
- 16.Liu E, Wu J, Cao W, et al. Curcumin induces G2/M cell cycle arrest in a p53-dependent manner and upregulates ING4 expression in human glioma. J Neuro-oncol. 2007;85(3):263–270. doi: 10.1007/s11060-007-9421-4. [DOI] [PubMed] [Google Scholar]
- 17.Kim TH, Jiang HH, Youn YS, et al. Preparation and characterization of water-soluble albumin-bound curcumin nanoparticles with improved antitumor activity. Int J Pharm. 2011;403(1):285–291. doi: 10.1016/j.ijpharm.2010.10.041. [DOI] [PubMed] [Google Scholar]
- 18.Shaikh J, Ankola DD, Beniwal V, Singh D, Kumar MR. Nanoparticle encapsulation improves oral bioavailability of curcumin by at least 9-fold when compared to curcumin administered with piperine as absorption enhancer. Eur J Pharm Sci. 2009;37(3):223–230. doi: 10.1016/j.ejps.2009.02.019. [DOI] [PubMed] [Google Scholar]
- 19.Narayanan NK, Nargi D, Randolph C, Narayanan BA. Liposome encapsulation of curcumin and resveratrol in combination reduces prostate cancer incidence in PTEN knockout mice. Int J Cancer. 2009;125(1):1–8. doi: 10.1002/ijc.24336. [DOI] [PubMed] [Google Scholar]
- 20.Mollazade M, Nejati-Koshki K, Akbarzadeh A, et al. PAMAM dendrimers augment inhibitory effects of curcumin on cancer cell proliferation: possible inhibition of telomerase. Asian Pac J Cancer Prev. 2013;14(11):6925–6928. doi: 10.7314/apjcp.2013.14.11.6925. [DOI] [PubMed] [Google Scholar]
- 21.Bisht S, Feldmann G, Soni S, et al. Polymeric nanoparticle-encapsulated curcumin (“nanocurcumin”): a novel strategy for human cancer therapy. J Nanobiotechnology. 2007;5(1):3. doi: 10.1186/1477-3155-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mangalathillam S, Rejinold NS, Nair A, Lakshmanan VK, Nair SV, Jayakumar R. Curcumin loaded chitin nanogels for skin cancer treatment via the transdermal route. Nanoscale. 2012;4(1):239–250. doi: 10.1039/c1nr11271f. [DOI] [PubMed] [Google Scholar]
- 23.Yadav VR, Prasad S, Kannappan R, et al. RETRACTED: Cyclodextrin-complexed curcumin exhibits anti-inflammatory and antiproliferative activities superior to those of curcumin through higher cellular uptake. Biochem Pharmacol. 2010;80(7):1021–1032. doi: 10.1016/j.bcp.2010.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 24.Sahu A, Bora U, Kasoju N, Goswami P. Synthesis of novel biodegradable and self-assembling methoxy poly (ethylene glycol)–palmitate nanocarrier for curcumin delivery to cancer cells. Acta Biomater. 2008;4(6):1752–1761. doi: 10.1016/j.actbio.2008.04.021. [DOI] [PubMed] [Google Scholar]
- 25.Li P, Dai YN, Zhang JP, Wang AQ, Wei Q. Chitosan-alginate nanoparticles as a novel drug delivery system for nifedipine. Int J Biomed Sci. 2008;4(3):221–228. [PMC free article] [PubMed] [Google Scholar]
- 26.De S, Robinson D. Polymer relationships during preparation of chitosan–alginate and poly-l-lysine–alginate nanospheres. J Control Release. 2003;89(1):101–112. doi: 10.1016/s0168-3659(03)00098-1. [DOI] [PubMed] [Google Scholar]
- 27.Murata Y, Jinno D, Liu D, Isobe T, Kofuji K, Kawashima S. The drug release profile from calcium-induced alginate gel beads coated with an alginate hydrolysate. Molecules. 2007;12(11):2559–2566. doi: 10.3390/12112559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chuah LH, Billa N, Roberts CJ, Burley JC, Manickam S. Curcumin-containing chitosan nanoparticles as a potential mucoadhesive delivery system to the colon. Pharm Dev Technol. 2013;18(3):591–599. doi: 10.3109/10837450.2011.640688. [DOI] [PubMed] [Google Scholar]
- 29.Mirnejad R, Jahromi MAM, Al-Musawi S, et al. Curcumin-loaded chitosan tripolyphosphate nanoparticles as a safe, natural and effective antibiotic inhibits the infection of Staphylococcus aureus and Pseudomonas aeruginosa in vivo. Iran J Biotechnol. 2014;12(3):1–8. [Google Scholar]
- 30.Siddique YH, Khan W, Singh BR, Naqvi AH. Synthesis of alginate-curcumin nanocomposite and its protective role in transgenic Drosophila model of Parkinson’s disease. ISRN Pharmacol. 2013;2013:794582. doi: 10.1155/2013/794582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saralkar P, Dash AK. Alginate nanoparticles containing curcumin and resveratrol: preparation, characterization, and in vitro evaluation against DU145 prostate cancer cell line. AAPS PharmSciTech. 2017 Apr 10; doi: 10.1208/s12249-017-0772-7. Epub. [DOI] [PubMed] [Google Scholar]
- 32.Das RK, Kasoju N, Bora U. Encapsulation of curcumin in alginate-chitosan-pluronic composite nanoparticles for delivery to cancer cells. Nanomedicine. 2010;6(1):153–160. doi: 10.1016/j.nano.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 33.Dai M, Zheng X, Xu X, et al. Chitosan-alginate sponge: preparation and application in curcumin delivery for dermal wound healing in rat. J BioMed Biotechnol. 2009;2009:595126. doi: 10.1155/2009/595126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Karri VV, Kuppusamy G, Talluri SV, et al. Curcumin loaded chitosan nanoparticles impregnated into collagen-alginate scaffolds for diabetic wound healing. Int J Biol Macromol. 2016;93:1519–1529. doi: 10.1016/j.ijbiomac.2016.05.038. [DOI] [PubMed] [Google Scholar]
- 35.Suryani, Halid NHA, Akib NI, Rahmanpiu, Mutmainnah N.Preparation of curcumin nanoparticle by using reinforcement ionic gelation technique Paper presented at: AIP Conference Proceedings20171838(1). [Google Scholar]
- 36.Caetano LA, Almeida AJ, Gonçalves LM, Effect of experimental parameters on alginate/chitosan microparticles for BCG encapsulation Mar Drugs 201614(5):pii: E 90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu J, Xu L, Liu C, et al. Preparation and characterization of cationic curcumin nanoparticles for improvement of cellular uptake. Carbohydr Polym. 2012;90(1):16–22. doi: 10.1016/j.carbpol.2012.04.036. [DOI] [PubMed] [Google Scholar]
- 38.Cheng KK, Chan PS, Fan S, et al. Curcumin-conjugated magnetic nanoparticles for detecting amyloid plaques in Alzheimer’s disease mice using magnetic resonance imaging (MRI) Biomaterials. 2015;44:155–172. doi: 10.1016/j.biomaterials.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 39.Ravindran J, Prasad S, Aggarwal BB. Curcumin and cancer cells: how many ways can curry kill tumor cells selectively? AAPS J. 2009;11(3):495–510. doi: 10.1208/s12248-009-9128-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dey S, Sreenivasan K. Conjugation of curcumin onto alginate enhances aqueous solubility and stability of curcumin. Carbohy Polym. 2014;99:499–507. doi: 10.1016/j.carbpol.2013.08.067. [DOI] [PubMed] [Google Scholar]
- 41.Portney NG, Ozkan M. Nano-oncology: drug delivery, imaging, and sensing. Anal Bioanal Chem. 2006;384(3):620–630. doi: 10.1007/s00216-005-0247-7. [DOI] [PubMed] [Google Scholar]
- 42.Akhtar F, Rizvi MM, Kar SK. Oral delivery of curcumin bound to chitosan nanoparticles cured Plasmodium yoelii infected mice. Biotechnol Adv. 2012;30(1):310–320. doi: 10.1016/j.biotechadv.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 43.Rejinold NS, Muthunarayanan M, Chennazhi KP, Nair SV, Jayakumar R. Curcumin loaded fibrinogen nanoparticles for cancer drug delivery. J Biomed Nanotechnol. 2011;7(4):521–534. doi: 10.1166/jbn.2011.1320. [DOI] [PubMed] [Google Scholar]
- 44.Taebnia N, Morshedi D, Yaghmaei S, Aliakbari F, Rahimi F, Arpanaei A. Curcumin-loaded amine-functionalized mesoporous silica nanoparticles inhibit α-synuclein fibrillation and reduce its cytotoxicity-associated effects. Langmuir. 2016;32(50):13394–13402. doi: 10.1021/acs.langmuir.6b02935. [DOI] [PubMed] [Google Scholar]
- 45.Choudhuri T, Pal S, Agwarwal ML, Das T, Sa G. Curcumin induces apoptosis in human breast cancer cells through p53-dependent Bax induction. FEBS Lett. 2002;512(1–3):334–340. doi: 10.1016/s0014-5793(02)02292-5. [DOI] [PubMed] [Google Scholar]
- 46.Azuma K, Osaki T, Minami S, Okamoto Y. Anticancer and anti-inflammatory properties of chitin and chitosan oligosaccharides. J Funct Biomater. 2015;6(1):33–49. doi: 10.3390/jfb6010033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wimardhani YS, Suniarti DF, Freisleben HJ, Wanandi SI, Siregar NC, Ikeda MA. Chitosan exerts anticancer activity through induction of apoptosis and cell cycle arrest in oral cancer cells. J Oral Sci. 2014;56(2):119–126. doi: 10.2334/josnusd.56.119. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.
Primer sequences
| Primer name | Sequence (5′→3′) | Size (bp) | Annealing temperature (°C) |
|---|---|---|---|
| GAPDH | Forward: GGTGGTCTCCTCTGACTTCAACA; reverse: GTTGCTGTAGCAAATTCGTTGT | 126 | 60 |
| Bax | Forward: TGG CAG CTG ACA TGT TTT CTG AC; reverse: TCA CCC AAC CAC CCT GGT CTT | 195 | 60 |
| Bcl2 | Forward: TCG CCC TGT GGATGA CTG A; reverse: CAGAGA CAG CCA GGA GAA ATC A | 134 | 60 |