Figure 1.
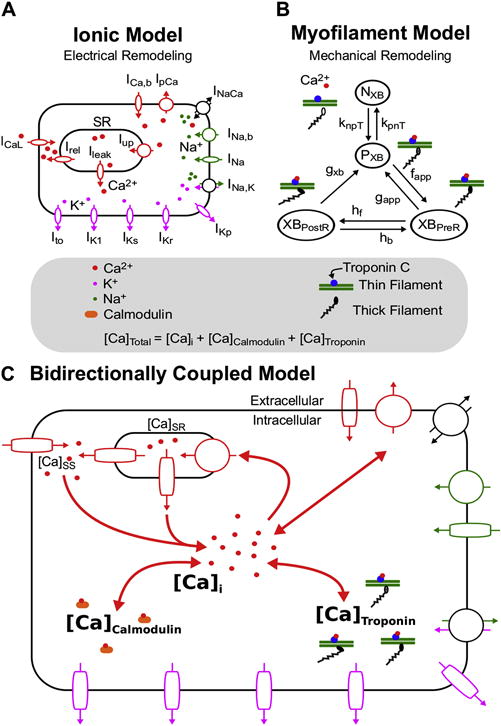
Ionic (A) and Myofilament (B) models combined to form the Bidirectionally Coupled Human Electrophysiology-Force Myocyte Model (C). A modified version of the ten Tusscher ionic model from Vandersickel et al (Vandersickel et al., 2014) was used for the ionic model. A modified version of the Markov state diagram of the myofilament model from Rice et al, which describes thin filament activation via free intracellular calcium ([Ca]i) binding to Troponin C as well as thin filament binding to thick filaments to form crossbridges (XBs), was used for the myofilament model. The transition rates (knpT and kpnT) between the thin filament states where XB formation is inhibited (NXB) and where weakly bound XB formation is possible (PXB) are both functions of perm50, kon, koffH, and koffL. The rate knpT is also dependent on kn_p, and kpnT is additionally dependent on kp_n. The XBPreR and XBPostR states represent a thin filament with a strongly bound XB that do not and do, respectively, have rotated myosin heads which induced strain (Rice et al., 2008). Bidirectional coupling was obtained by incorporating MEF on calcium dynamics via Equation 1, where the total cytoplasmic calcium ([Ca]Total) is equal to the sum of [Ca]i, the total calcium bound to calmodulin ([Ca]Calmodulin), and the total calcium bound to troponin C ([Ca]Troponin).
