Abstract
Epidermal squamous cell carcinoma is an extremely common type of cancer. Early tumors can be successfully treated by surgery, but recurrent disease is aggressive and resistant to therapy. Cisplatin is often used as a treatment, but the outcome is rarely satisfactory. For this reason new strategies are required. Sulforaphane is a diet-derived cancer prevention agent that is effective in suppressing tumor growth in animal models of skin cancer. We monitored the efficacy of sulforaphane and cisplatin as a combined therapy for squamous cell carcinoma. Both agents suppress cell proliferation, growth of cancer stem cell spheroids, matrigel invasion and migration of SCC-13 and HaCaT cells and combination treatment is more efficient. In addition, SCC-13 cell derived cancer stem cells are more responsive to these agents than non-stem cancer cells. Both agents suppress tumor formation, but enhanced suppression is observed with combined treatment. Moreover, both agents reduce the number of tumor-resident cancer stem cells. SFN treatment of cultured cells or tumors increases apoptosis and p21Cip1 level, and both agents increase tumor apoptosis. We suggest that combined therapy with sulforaphane and cisplatin is efficient in suppressing tumor formation and may be a treatment option for advanced epidermal squamous cell carcinoma.
Keywords: Sulforaphane, SFN, squamous cell carcinoma, cisplatin, HaCaT, SCC-13, cancer stem cells
Introduction
Epidermal squamous cell carcinoma is a leading cause of cancer that is routinely treated by surgical resection [1]. However, nearly 10% of these cancers recur as highly aggressive and invasive cancers that must be treated with chemotherapy [2,3]. Cisplatin, doxorubicin, 5-fluorouracil and bleomycin have been used to treat this disease [2]; however, chemoresistance is an important problem [2,4]. Designing strategies to overcome chemotherapy resistance is an important goal. The clinical scenario suggests that a subset of tumor cells are unaffected by therapy and are able to initiate formation of a new tumor. Considering that tumors are complex “organs” comprising many tumor cell variants, it is plausible that therapy resistance is related to a subpopulation of less differentiated, multipotent, self-renewing and aggressive cancer stem cells [5,6]. Current therapies often target the highly proliferative tumor cells to reduce tumor bulk, but these agents do not always kill cancer stem cells [7–10].
Squamous cell carcinoma contains a population of cells that are able to self-renew [11–14]. Our recent studies confirm that a highly aggressive subpopulation of cancer cells, epidermal cancer stem cells (ECS cells), are present in tumors and epidermis-derived skin cancer cell lines [15–17]. Therapies targeting cancer stem cells, in combination with conventional chemotherapy agents, may improve skin cancer treatment and reduce cancer recurrence. We have shown that SFN, an important diet-derived candidate cancer prevention agent [18], induces loss of expression of proteins associated with cancer stem cell survival [19–21]. These studies suggest that SFN may be a useful co-therapy in conjunction with agents that kill bulk tumor cells. Moreover, SFN has minimal side effects when administered to patients or mice, and is highly soluble and bioavailable in vivo [22–24]. In the present study we examine the impact of co-treatment with SFN and cisplatin on tumor cells and show that these agents act together to suppress cell proliferation, stem cell spheroid formation, invasion, migration and tumor formation.
Materials and Methods
Antibodies and reagents
DMEM (11960-077), sodium pyruvate, (11360-070), L-Glutamine (25030-164), penicillin-streptomycin solution (15140-122) and 0.25% trypsin-EDTA (25200-056) were purchased from Gibco (Grand Island, NY). Heat-inactivated fetal calf serum (FCS, F4135) was obtained from Sigma. Anti-β-actin (A5441) was purchased from Sigma (St. Louis, MO). Procaspase-9 (9502), procaspase-8 (9746) and procaspase-3 (9665) antibodies were from Cell Signaling (Danvers, MA) and the PARP antibody (556494) was from BD Pharmingen (San Diego, CA). Anti-p21Cip1 was obtained from Cell Signaling (2947, Danvers, MA). Alexa Fluor 594-conjugated goat anti-rat IgG (A11007), Alexa Fluor 488-conjugated goat anti-mouse IgG (A21121) and Alexa Fluor 594-conjugated goat anti-rabbit IgG (A11012) secondary antibodies were obtained from Invitrogen and used at 1:500 dilution. Peroxidase conjugated anti-mouse IgG (NXA931) and anti-rabbit IgG (NA934V) were obtained from GE Healthcare (Buckinghamshire, UK) and used at a 1:5000 dilution. Sulphoraphane (S8044, SFN) was obtained from LKT Laboratories, Inc. (St. Paul, Minnesota) and stocks were prepared in dimethyl sulfoxide as in our previous report [25]. Cisplatin (100351) was purchased from APP Pharmaceuticals, a division of Fresenius Kabi USA (Lake Zurich, IL), and stocks were prepared in Dulbecco’s phosphate buffered saline (21-031-CV, Corning Inc., Manassas, VA). BD Biocoat cell inserts (353097) and Matrigel (354234) were purchased from BD Biosciences. Statistical comparisons were made using the t-test.
Spheroid formation assay
SCC-13 and HaCaT cells were maintained in growth medium containing Dulbecco’s Modified Eagle’s Medium (DMEM, Invitrogen, Frederick, MD) supplemented with 4.5 mg/ml D-glucose, 200 mM L-glutamine, 100 µg/ml sodium pyruvate, 100 U/ml penicillin, 100 U/ml streptomycin and 5% fetal calf serum. For spheroid formation assay, 80% confluent cultures were harvested with trypsin and gently pipetted to form a single cell suspension. Trypsin was inactivated by addition of serum-containing medium and the cells were collected by centrifugation. The cells were resuspended in spheroid medium which is DMEM/F12 (1:1) (DMT-10-090-CV, Mediatech Inc, Manassa, VA) containing 2% B27 serum-free supplement (17504-044, Invitrogen, Frederick, MD), 20 ng/ml EGF (E4269, Sigma, St. Louis), 0.4% bovine serum albumin (B4287, Sigma) and 4 µg/ml insulin (Sigma, St. Louis, MO, #19278), and plated at 40,000 cells per 9.5 cm2 well in six-well ultra-low attachment cluster dishes (#3471, Corning, Tewksbury, MA). For assay of SFN and cisplatin impact spheroids were permitted to grow for 8 d. SFN or cisplatin treatment was then initiated and spheroid number was monitor daily thereafter [15].
Immunoblot
For immunoblot, equivalent amounts of protein were electrophoresed on denaturing and reducing 8% polyacrylamide gels and transferred to nitrocellulose membrane. The membrane was blocked by 5% nonfat dry milk and then incubated with the appropriate primary (1:1000) and secondary antibody (1:5000). Secondary antibody binding was visualized using chemiluminescence detection technology.
Proliferation assay
SCC-13 cells were grown for one week as monolayers in spheroid media. Cells were harvested with 0.25% trypsin, resuspended in spheroid medium and grown as monolayer cultures. At 24 h after plating, treatment was initiated with SFN or cisplatin or appropriate vehicle. Cells were harvested at various times and cell number was counted using a Z1 Coulter Particle Counter (Beckman Coulter).
Invasion assay
Matrigel was diluted in 0.01 M Tris-HCl/0.7% NaCl, filter sterilized and 0.1 ml was used to coat individual BD BioCoat inserts (Millicell-PCF, 0.4 µm, 12 mm, PIHP01250). Cells (25,000) were plated in 100 µl spheroid medium, supplemented with 2% FCS, in the upper chamber atop the matrigel. The lower chamber contained spheroid medium supplemented with 10% FCS. After 18 h, the membranes were harvested and excess cells were removed from the upper membrane surface. The membrane was fixed in 4% paraformaldehyde, stained with 1 µg/ml DAPI, and the underside of the membrane was photographed with an inverted fluorescent microscope and the number of cells counted.
Migration assay
SCC-13 cells (2 × 106) were plated in 10 cm dishes and grown as monolayer cultures in spheroid medium until confluent. A 10 µl pipette tip was used to prepare uniform areas void of cells and the dishes were washed to remove the dislodged cells. Images were collected at 0 – 24 h after the scratch using the 4 × objective, and the width of the opening was measured as a function of time as an index of cell migration potential.
Tumor xenograft growth assays
Cancer cells were trypsinized to prepare a single cell suspension and resuspended in phosphate buffered saline containing 30% Matrigel, and 100 µl of solution containing 100,000 cells was injected subcutaneously into the two front flanks in NSG (NOD/scid/IL-2 receptor gamma knockout) mice using a 26.5 gauge needle. Four mice were used per data point (two tumors per mouse). Cisplatin was dissolved in sterile phosphate buffered saline (PBS) for IP injection at 2 mg per kg body. Cisplatin treatment was initiated two days after tumor cell injection and repeated every two weeks [26]. SFN was dissolved in sterile water and delivered by oral gavage three times per week (M/W/F) at 20 µmoles/dose beginning two days after tumor cell injection. Tumor growth was monitored by measuring tumor diameter and calculating tumor volume [27] and tumors were harvested a 5 wks. Mice were euthanized and tumor samples were harvested to prepare extracts for immunoblot. These experiments were reviewed and approved by the University of Maryland Baltimore Institutional Animal Care and Use Committee.
Tumor-derived cell spheroid formation assay
Tumors were collected in 50 ml conical in 10 ml Keratinocyte serum-free medium (KSFM) containing 200 U/ml penicillin, 200 µg/ml streptomycin, 7.5 µg/ml gentamycin, and 0.25 µg/ml of Fungizone (Gibco, G15290-08, Gaithersburg, MD), and incubated overnight at 4 C. The next day, the tumors were rinsed with Hank’s Balance Salt Solution (Ca++/Mg++-free) and mechanically dissociated before transferring to new 50 ml conical and incubating with 5 ml of 0.25% Trypsin-EDTA for 10 min at 37 °C. Serum containing medium was added to inhibit trypsin, the samples were centrifuged for 5 min, and the supernatant was removed. The dissociated tumor cells were resuspended in spheroid medium and filtered through 40-µm pore size cell strainer. The final cell suspensions were counted and 20,000 trypan blue-positive viable cells were plated per well in a 6 well low-attachment plates in the absence of cisplatin or SFN. Spheroid formation was monitored from 0 – 8 d. A minimum of three tumors were tested per each treatment, and spheroid growth from each tumor was monitored in each of three individual wells.
Results
Enhanced sensitivity of ECS cells to cisplatin and SFN
ECS cells display increased spheroid formation, migration and invasion, and form large, highly vascularized and aggressive tumors as compared to non-stem cancer cells [15,28–31]. Since these cells drive tumor recurrence, metastasis and resistance to chemotherapy, they are important therapy targets. We first compare the response of non-stem cancer cells and ECS cells to treatment with SFN and cisplatin.
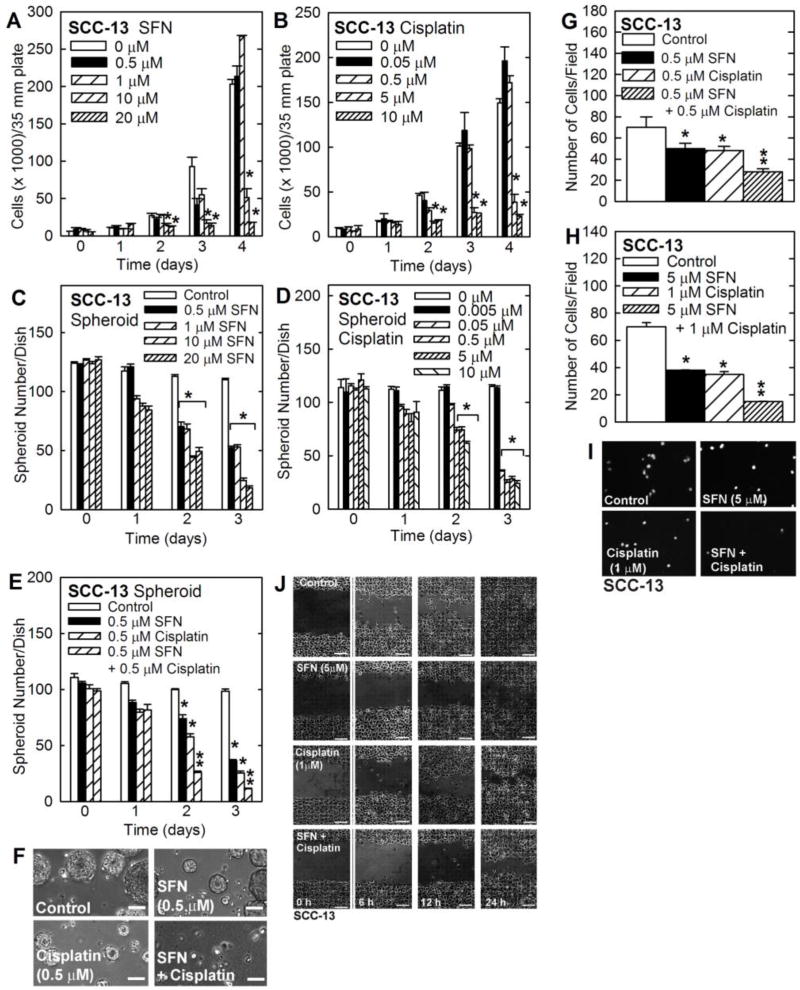
Monolayer SCC-13 cultures are comprised largely of non-stem cancer cells [15]. Fig. 1A/B shows that SFN and cisplatin markedly suppress SCC-13 cell proliferation at concentrations of 10 µM and 5 µM, respectively. Growth of monolayer cells on ultra-low attachment plates selects for cells that grow as free-floating spheroids and display stem cell properties [15]. Most of the cells plated in spheroid growth conditions survival less than 12 h, and only 0.15% have the potential to survive and form spheroids [15]. Fig. 1C/D shows that SFN and cisplatin suppress SCC-13 spheroid formation at concentrations of 0.5 µM and 0.05 µM, respectively. Thus, SFN and cisplatin are more effective at suppressing spheroid number than monolayer growth. In addition, combined treatment with 0.5 µM SFN and 0.5 µM cisplatin produces a more dramatic reduction in spheroid number than treatment with each individual agent (Fig. 1E/F).
Fig. 1. Impact of SFN and cisplatin on cell function.
A/B SCC-13 cells, growing in monolayer culture in spheroid medium, were treated as indicated and cell number was determined at the indicated times. C/D/E/F SCC-13 cell-derived eight day pre-formed spheroids were treated with cisplatin or SFN for 0 – 3 d and spheroid number per dish was determined. G/H/I SCC-13 cells were seeded atop a layer of matrigel in a BD BioCoat chamber in the presence of the indicated agents and matrigel invaded cells were imaged and counted at 18 h. J Confluent monolayers of SCC-13 cells were uniformly wounded with a pipette tip and wound closure was monitored from 0 – 24 h. The graphical values are mean ± SEM, n = 3. The asterisks indicate a significant change compared to control, p < 0.001. The double asterisks indicate a significant reduction compare to the single treatment groups, p < 0.005.
Impact of SFN and cisplatin on ECS cell invasion and migration
ECS cell invasion and migration, are enhanced in ECS cells as compared to non-stem cancer cells [15,17]. We therefore next monitored the impact of cisplatin and SFN on ECS cell invasion and migration. Cells (25,000) were plated in a Transwell chamber atop a matrigel-coated membrane and then treated with 0.5 µM SFN, 0.5 µM cisplatin or both agents. After 18 h, the membrane was stained to detect cells that had invaded through the matrigel to the lower chamber. Fig. 1G shows that SFN and cisplatin reduce cell invasion, and that combined treatment further reduces invasion. We repeated this experiment using higher concentrations of each agent. Fig. 1H/I shows that 5 µM SFN and 1 µM cisplatin produce a more substantial reduction in matrigel invasion. However, the combination treatment is more efficient. To study cell migration, ECS cells were seeded at confluent density in monolayer culture, wounds were created by scraping with a pipette tip and wound closure was monitored from 0 to 24 h. As shown in Fig. 1J, cisplatin and SFN treatment reduce wound closure as does combined treatment.
Impact of SFN and cisplatin on tumor formation
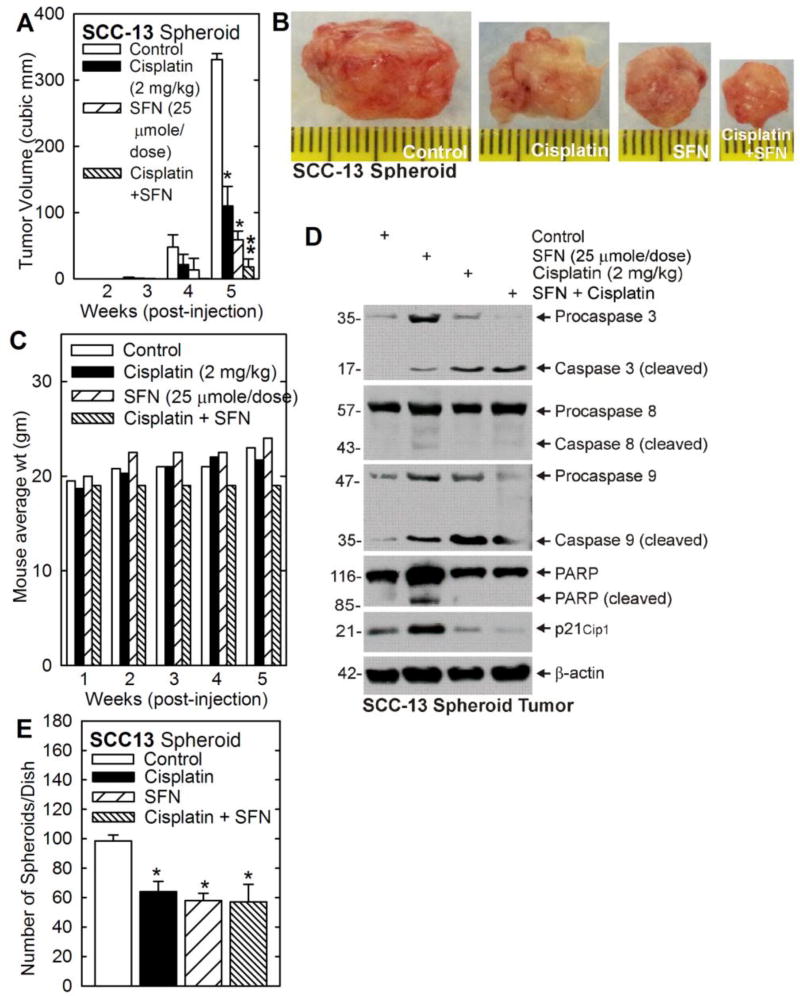
We next examined the impact of cisplatin and SFN on tumor formation. SCC-13 cell-derived ECS cells were injected at 100,000 per each front flank in NSG mice and treatment was initiated with cisplatin, SFN or the combination. Fig. 2A/B shows that cisplatin and SFN reduce tumor size and the combination produces a more substantial reduction. Mouse body weight increases slightly from 20 to 23 g in all groups except for the combination treatment where body weight remains stable (Fig. 2C). Examination of tumor cell death markers reveals that cleavage of procaspase-3 and -9 and PARP is increased in both SFN and cisplatin treated cells (Fig. 2D). Procaspase-8 cleavage, in contrast, is not markedly changed. In addition, p21Cip1 levels are increased in SFN but not cisplatin treated groups.
Fig. 2. SFN and cisplatin impact on tumor formation by SCC-13 spheroid-derived cells.
A/B/C SCC-13 spheroid cells were injected subcutaneously into immunocompromised NSG mice and at 48 h treatment was initiated with the indicated levels of SFN and cisplatin. Treatments were selected such that the individual treatments produce a partial reduction in tumor formation. Tumor size and morphology was monitored and tumor samples were harvested a 5 wk. Mouse weight was normal for the duration of the experiment in all treatment groups. The reduced weight gain in the cisplatin/SFN group was not significant different compared to the other treatment groups. D SFN and cisplatin treatment induces tumor cell apoptosis. Tumor extracts were prepared at 5 wk for immunoblot detection of the indicated epitopes. E Tumors were harvested and cells were dispersed as single cell suspensions. Twenty thousand viable cells were seeded in spheroid growth conditions and the number of spheroids formed was monitored at 5 d. All graphical values are mean ± SEM, n = 5. The asterisks indicate a significant change compared to control, p < 0.001. The double asterisks indicate a significant reduction compare to the single treatment groups, p < 0.005.
To assess the cancer stem cell status of the tumor cells following cisplatin-, SFN- and combination-treatment, tumor cells were harvested, dissociated and monitored for ability to form spheroids in the absence of drug treatment. The number of spheroids formed in this assay is an index of the number of cancer stem-like cells in the tumor [32–34]. Equal numbers of tumor-derived single cells were plated on ultralow attachment plates and spheroid formation was monitored. Fig. 2E shows that cells from non-treated tumors produce a significant number of spheroids and that this number is equally reduced by SFN, cisplatin and combination treatment. These findings suggest that both agents reduce the number of cells displaying stem cell-like biological behavior.
Impact of cisplatin and SFN on HaCaT cells
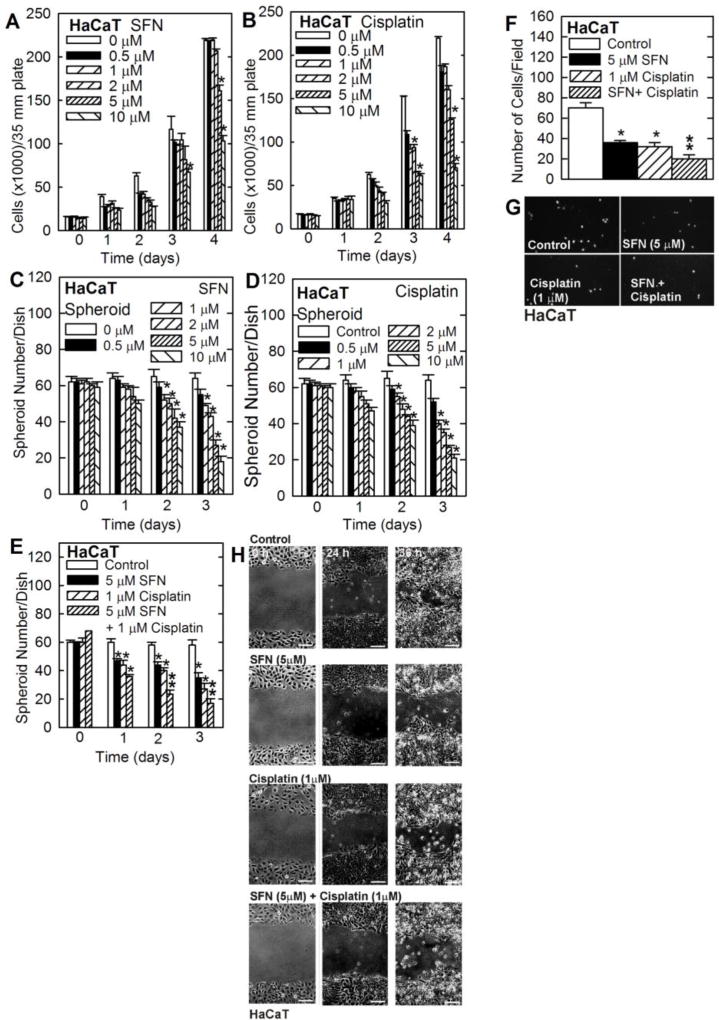
To assess whether SFN and cisplatin produce similar responses in other cell lines, we examined the impact of these agents on HaCaT cells. HaCaT cells are an immmortalized, but non-tumorigenic epidermis-derived cell line [35]. A substantial reduction in HaCaT cells survival was observed following treatment with 5 to 10 µM of SFN or cisplatin (Fig. 3A/B). A similar reduction in spheroid formation was observed following treatment with either agent (Fig. 3C/D). In addition, treatment of HaCaT cells with 5 µM SFN or 1 µM cisplatin reduced spheroid formation and combination treatment produced an additional reduction (Fig. 3E). We next monitored the impact on cell invasion and migration. Fig. 3F/G shows that both agents suppress matrigel invasion and that combined treatment further suppresses invasion. Fig. 3G shows that each agent suppresses HaCaT cell migration.
Fig. 3. Impact of SFN and cisplatin on cell function.
A/B HaCaT cells, growing in monolayer culture, were treated as indicated and cell number was determined at the indicated times. C/D/E HaCaT cell-derived eight day pre-formed spheroids were treated with cisplatin or SFN for 0 – 3 d and spheroid number per dish was determined. F/G HaCaT cells were seeded atop a layer of matrigel in a BD BioCoat chamber in the presence of the indicated agents and matrigel invaded cells were imaged and counted at 18 h. H Confluent monolayers of HaCaT cells were wounded with a pipette tip and wound closure was monitored rom 0 – 24 h. The graphical values are mean ± SEM, n = 3. The asterisks indicate a significant change compared to control, p < 0.001. The double asterisks indicate a significant reduction compare to the single treatment groups, p < 0.005.
Impact of SFN and cisplatin on apoptosis and ECS cell survival markers
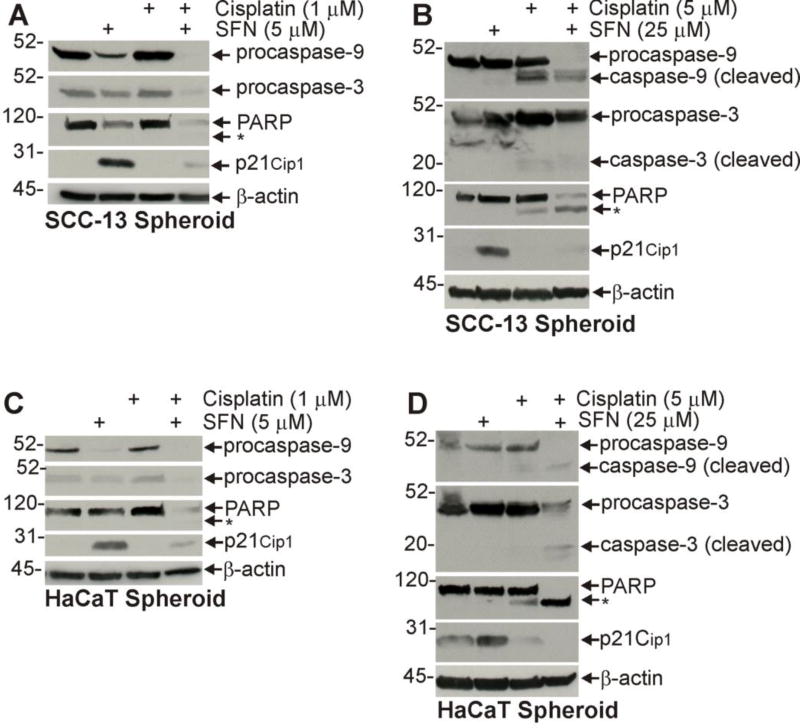
Fig. 2D shows that SFN or cisplatin treatment activates procaspase 9, procaspase 3, and PARP cleavage and that this is associated with increased apoptosis in ECS cell-derived tumors. We next determined whether a similar pattern of caspase cleavage was observed in SFN- and cisplatin-treated cultured ECS cells. Cells were grown as spheroids for 5 d and then treated with SFN or cisplatin for 72 h before extracts were prepared for immunoblot. Fig. 4A shows that a SFN treatment of SCC-13 ECS cells leads to reduced procaspase-9, procaspase-3 and PARP level, and increased p21Cip1 and that treating with higher concentrations of these agents leads to appearance of procaspase and PARP cleavage products (Fig. 4B). A similar pattern of change is observed in HaCaT cells treated with low versus high levels of cisplatin and SFN (Fig. 4C/D). It is interesting that p21Cip1 level is markedly increased by SFN treated cells, but not in cisplatin or combination-treated cells (Fig. 4A/B/C/D). These finding are consistent with the changes observed in SFN and cisplatin treated tumors.
Fig. 4. Impact of SFN and cisplatin treatment on apoptosis in ECS cell enriched spheroid cultures.
A/B/C/D SCC-13 or HaCaT cell spheroids were grown for 5 d and then treated with SFN and/or cisplatin for 72 h before preparation of extracts for immunoblot. Similar results were observed in each of three experiments. The asterisk denotes cleaved PARP.
Discussion
Cisplatin, SFN and skin cancer
Epidermal squamous cell carcinoma can be successfully treated by surgical excision, but this is not effective for high grade (grade 4) tumors [1]. In fact, 55% of grade 4 disease is not cured by Moh’s surgery and requires cytotoxic systemic chemotherapy. Cisplatin is a DNA intercalating agent that is frequently used to treat recurrent epidermal squamous cell carcinoma [1] and combination therapy, for example with cisplatin and 5-fluorouracil, is also an option [1]. However, these approaches are not satisfactory and so additional treatment options are needed.
SFN is an important cancer prevention/therapy agent derived from cruciferous vegetables (broccoli, etc.) [18,36,37]. SFN was selected for the present studies because it has efficacy against skin cancer in several model systems [36,36,38], has high bioavailability in vivo [22] and is known to protect against skin cancer induction [23,36,38]. Moreover, it can be detected at bioactive levels in blood and tissues of broccoli-fed patients confirming that biologically relevant levels can be achieved [23].
Impact of SFN and cisplatin on epidermis-derived cell lines
Dose response studies reported herein indicate that SFN and cisplatin, at 10 µM and 5 µM, respectively, suppress growth of monolayer SCC-13 cell cultures, which we previously showed are comprised of greater than 99% non-stem cancer cells [15],. In contrast, we show that ECS cell spheroid formation is suppressed at SFN and cisplatin at lower concentrations of 0.5 µM and 0.005 µM, respectively. Spheroids, are highly enriched in ECS cells (15%) and display cancer stem cell-associated features including enhanced ability to invade matrigel, migrate and undergo EMT [15,17,29,30]. They also form rapidly growing, highly aggressive, invasive and vascularized tumors compared to non-stem cancer cells [15,29]. Moreover, further studies reveal that combination treatment more effectively reduces spheroid formation and matrigel invasion, showing that ECS cells are more sensitive to combination treatment. In addition, combination treatment, with higher levels of each agent (5 µM SFN, 1 µM cisplatin), produces more efficient suppression of invasion when compared to either agent alone. Although we do not understand the mechanism, it is interesting that SFN is more efficient at suppressing SCC-13 cell migration compared to cisplatin.
HaCaT cells are immortalized epidermis-derived cells that do not form tumors [39]. Although these cells do not form tumors, we have previously shown that they are capable of spheroid formation [16,17]. Treatment of HaCaT monolayer or spheroid cells with SFN or cisplatin (0 – 10 µM) produced a dose-dependent reduction in cell number. HaCaT-derived ECS cells are slightly more sensitive to these agents than non-stem cancer (monolayer) cells. In addition, combined cisplatin and SFN combined treatment further suppress HaCaT cell spheroid formation and matrigel invasion compared to either agent alone.
We have shown that SFN induces apoptosis and increases p21Cip1 level in epidermal cancer cells [19,40]. We confirm this finding in the present study and further show that ECS cells are more responsive than non-stem cancer cells. Moreover, the increased sensitivity to SFN is associated with increased procaspase-9, procaspase-3 and PARP cleavage and increased p21Cip1 level. The finding that SFN treatment increases p21Cip1 level and G2/M arrest is consistent with observations in prostate cancer [41,42], epidermal squamous cell carcinoma [19] and keratinocytes [25]. Cisplatin, in contrast, did not impact p21Cip1 level, but did inhibit the SFN-dependent increase in p21Cip1. It is not clear why cisplatin treatment inhibits the SFN-dependent increase in p21Cip1, as cisplatin has been reported to increase p21Cip1 in cancer cells [43]. However, it is possible that cisplatin may permit these cells to escape G2/M arrest leading to premature entry into mitosis and death [43]. In this context, the cells would not arrest in G2/M and p21Cip1 accumulation would not be observed.
Impact of SFN and cisplatin on tumor formation
Tumor xenograft studies show that cisplatin and SFN reduce tumor formation. Cisplatin (2 mg/kg) and SFN (25 µmole/dose) markedly reduce tumor formation in the SCC-13 cell xenograft model, and combined treatment further reduces tumor size. Biochemical analysis of the tumors shows that each agent induces procaspase-9 and procaspase-3 cleavage (apoptosis). The level of cleaved PARP is similar in all groups, except in the SFN alone group where overall PARP and cleaved PARP levels are increased. p21Cip1 level is increased in SFN-treated tumors and co-treatment with cisplatin antagonizes this increase. This pattern of regulation of p21Cip1 by SFN and cisplatin is similar to that observed in cultured cells, and suggests that the SFN induction of p21Cip1 level may be partially antagonized by cisplatin.
We also monitored the impact of SFN and cisplatin treatment on tumor stem cell properties of the tumor cells. We harvested untreated, SFN, cisplatin, and combination treated tumors, dissociated the cells, and examined the ability of the cells to form spheroids in culture in the absence of treatment. This strategy measures the stem cell potential of the tumor-derived cells [32–34]. These studies show that both cisplatin and SFN reduce spheroid number suggesting that these agents deplete the ECS cell population in the tumor. This is in contrast to some studies which suggest that treatment with agents like cisplatin can positively enrich for cancer stem cells [32–34]. However, it should be noted that in many studies cisplatin selection of cancer stem cells required serial passage of the cancer cells in mice under continuous drug selective pressure [32–34].
SFN is a promising candidate agent to consider for co-therapy. It has efficacy against skin cancer in several model systems [36,36,38], is highly bioavailable in vivo [22], can protect against skin cancer induction and has no known side effects [23,36,38]. Our findings suggest that SFN/cisplatin combination may be a useful therapy in squamous cell carcinoma.
Acknowledgments
This work was supported by National Institutes of Health grants to RLE (R21 AR065266, R01 CA131074, R21 AR065226 and R01 CA184027). We thank the Greenebaum Comprehensive Cancer Center for provision of core facilities (P30 CA134274).
Footnotes
Conflict of Interest: The authors indicate no conflict of interest
References
- 1.Cranmer LD, Engelhardt C, Morgan SS. Treatment of unresectable and metastatic cutaneous squamous cell carcinoma. Oncologist. 2010;15:1320–1328. doi: 10.1634/theoncologist.2009-0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Antonyak MA, Li B, Boroughs LK, Johnson JL, Druso JE, Bryant KL, Holowka DA, Cerione RA. Cancer cell-derived microvesicles induce transformation by transferring tissue transglutaminase and fibronectin to recipient cells. Proc Natl Acad Sci U S A. 2011;108:4852–4857. doi: 10.1073/pnas.1017667108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shin DM, Glisson BS, Khuri FR, Clifford JL, Clayman G, Benner SE, Forastiere AA, Ginsberg L, Liu D, Lee JJ, Myers J, Goepfert H, Lotan R, Hong WK, Lippman SM. Phase II and biologic study of interferon alfa, retinoic acid, and cisplatin in advanced squamous skin cancer. J Clin Oncol. 2002;20:364–370. doi: 10.1200/JCO.2002.20.2.364. [DOI] [PubMed] [Google Scholar]
- 4.Miller DL, Weinstock MA. Nonmelanoma skin cancer in the United States: incidence. J Am Acad Dermatol. 1994;30:774–778. doi: 10.1016/s0190-9622(08)81509-5. [DOI] [PubMed] [Google Scholar]
- 5.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 6.Gupta PB, Chaffer CL, Weinberg RA. Cancer stem cells: mirage or reality? Nat Med. 2009;15:1010–1012. doi: 10.1038/nm0909-1010. [DOI] [PubMed] [Google Scholar]
- 7.Korkaya H, Kim GI, Davis A, Malik F, Henry NL, Ithimakin S, Quraishi AA, Tawakkol N, D'Angelo R, Paulson AK, Chung S, Luther T, Paholak HJ, Liu S, Hassan KA, Zen Q, Clouthier SG, Wicha MS. Activation of an IL6 inflammatory loop mediates trastuzumab resistance in HER2+ breast cancer by expanding the cancer stem cell population. Mol Cell. 2012;47:570–584. doi: 10.1016/j.molcel.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu YP, Yang CJ, Huang MS, Yeh CT, Wu AT, Lee YC, Lai TC, Lee CH, Hsiao YW, Lu J, Shen CN, Lu PJ, Hsiao M. Cisplatin selects for multidrug-resistant CD133+ cells in lung adenocarcinoma by activating Notch signaling. Cancer Res. 2013;73:406–416. doi: 10.1158/0008-5472.CAN-12-1733. [DOI] [PubMed] [Google Scholar]
- 9.Chen J, Li Y, Yu TS, McKay RM, Burns DK, Kernie SG, Parada LF. A restricted cell population propagates glioblastoma growth after chemotherapy. Nature. 2012;488:522–526. doi: 10.1038/nature11287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Visvader JE, Lindeman GJ. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat Rev Cancer. 2008;8:755–768. doi: 10.1038/nrc2499. [DOI] [PubMed] [Google Scholar]
- 11.Boehnke K, Falkowska-Hansen B, Stark HJ, Boukamp P. Stem cells of the human epidermis and their niche: Composition and function in epidermal regeneration and carcinogenesis. Carcinogenesis. 2012 doi: 10.1093/carcin/bgs136. [DOI] [PubMed] [Google Scholar]
- 12.Muffler S, Stark HJ, Amoros M, Falkowska-Hansen B, Boehnke K, Buhring HJ, Marme A, Bickenbach JR, Boukamp P. A stable niche supports long-term maintenance of human epidermal stem cells in organotypic cultures. Stem Cells. 2008;26:2506–2515. doi: 10.1634/stemcells.2007-0991. [DOI] [PubMed] [Google Scholar]
- 13.Eckert RL, Adhikary G, Balasubramanian S, Rorke EA, Vemuri MC, Boucher SE, Bickenbach JR, Kerr C. Biochemistry of epidermal stem cells. Biochim Biophys Acta. 2013;1830:2427–2434. doi: 10.1016/j.bbagen.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Singh A, Park H, Kangsamaksin T, Singh A, Readio N, Morris RJ. Keratinocyte Stem Cells and the Targets for Nonmelanoma Skin Cancer. Photochem Photobiol. 2012 doi: 10.1111/j.1751-1097.2012.01079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adhikary G, Grun D, Kerr C, Balasubramanian S, Rorke EA, Vemuri M, Boucher S, Bickenbach JR, Hornyak T, Xu W, Fisher ML, Eckert RL. Identification of a population of epidermal squamous cell carcinoma cells with enhanced potential for tumor formation. PLoS One. 2013;8:e84324. doi: 10.1371/journal.pone.0084324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fisher ML, Keillor JW, Xu W, Eckert RL, Kerr C. Transglutaminase is required for epidermal squamous cell carcinoma stem cell survival. Mol Cancer Res. 2015;13:1083–1094. doi: 10.1158/1541-7786.MCR-14-0685-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fisher ML, Adhikary G, Xu W, Kerr C, Keillor JW, Eckert RL. Type II transglutaminase stimulates epidermal cancer stem cell epithelial-mesenchymal transition. Oncotarget. 2015;6:20525–20539. doi: 10.18632/oncotarget.3890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clarke JD, Dashwood RH, Ho E. Multi-targeted prevention of cancer by sulforaphane. Cancer Lett. 2008;269:291–304. doi: 10.1016/j.canlet.2008.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Balasubramanian S, Chew YC, Eckert RL. Sulforaphane Suppresses Polycomb Group Protein Level via a Proteasome-Dependent Mechanism in Skin Cancer Cells. Mol Pharmacol. 2011;80:870–878. doi: 10.1124/mol.111.072363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Balasubramanian S, Kanade S, Han B, Eckert RL. A proteasome inhibitor-stimulated Nrf1-dependent compensatory increase in proteasome subunit gene expression reduces polycomb group protein level. J Biol Chem. 2012;287:36179–36189. doi: 10.1074/jbc.M112.359281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choudhury SR, Balasubramanian S, Chew YC, Han B, Marquez VE, Eckert RL. (−)-Epigallocatechin-3-gallate and DZNep reduce polycomb protein level via a proteasome-dependent mechanism in skin cancer cells. Carcinogenesis. 2011;32:1525–1532. doi: 10.1093/carcin/bgr171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fimognari C, Hrelia P. Sulforaphane as a promising molecule for fighting cancer. Mutat Res. 2007;635:90–104. doi: 10.1016/j.mrrev.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 23.Xu C, Huang MT, Shen G, Yuan X, Lin W, Khor TO, Conney AH, Kong AN. Inhibition of 7,12-dimethylbenz(a)anthracene-induced skin tumorigenesis in C57BL/6 mice by sulforaphane is mediated by nuclear factor E2-related factor 2. Cancer Res. 2006;66:8293–8296. doi: 10.1158/0008-5472.CAN-06-0300. [DOI] [PubMed] [Google Scholar]
- 24.Egner PA, Chen JG, Zarth AT, Ng DK, Wang JB, Kensler KH, Jacobson LP, Munoz A, Johnson JL, Groopman JD, Fahey JW, Talalay P, Zhu J, Chen TY, Qian GS, Carmella SG, Hecht SS, Kensler TW. Rapid and sustainable detoxication of airborne pollutants by broccoli sprout beverage: results of a randomized clinical trial in China. Cancer Prev Res (Phila) 2014;7:813–823. doi: 10.1158/1940-6207.CAPR-14-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chew YC, Adhikary G, Wilson GM, Xu W, Eckert RL. Sulforaphane induction of p21(Cip1) cyclin-dependent kinase inhibitor expression requires p53 and Sp1 transcription factors and is p53-dependent. J Biol Chem. 2012;287:16168–16178. doi: 10.1074/jbc.M111.305292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Su X, Dong C, Zhang J, Su L, Wang X, Cui H, Chen Z. Combination therapy of anti-cancer bioactive peptide with Cisplatin decreases chemotherapy dosing and toxicity to improve the quality of life in xenograft nude mice bearing human gastric cancer. Cell Biosci. 2014;4:7. doi: 10.1186/2045-3701-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Streit M, Velasco P, Brown LF, Skobe M, Richard L, Riccardi L, Lawler J, Detmar M. Overexpression of thrombospondin-1 decreases angiogenesis and inhibits the growth of human cutaneous squamous cell carcinomas. Am J Pathol. 1999;155:441–452. doi: 10.1016/S0002-9440(10)65140-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eckert RL, Fisher ML, Grun D, Adhikary G, Xu W, Kerr C. Transglutaminase is a tumor cell and cancer stem cell survival factor. Mol Carcinog. 2015;54:947–958. doi: 10.1002/mc.22375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fisher ML, Kerr C, Adhikary G, Grun D, Xu W, Keillor JW, Eckert RL. Transglutaminase interaction with α6/β4-integrin to stimulates YAP1-dependent ΔNp63α stabilization and leads to enhanced cancer stem cell survival and tumor formation. Cancer Res. 2016;76:7265–7276. doi: 10.1158/0008-5472.CAN-16-2032. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 30.Fisher M, Adhikary G, Grun D, Kaetzel D, Eckert R. The Ezh2 polycomb group p rotein drives an aggressive phentype in melanoma cancer stem cells and is a target of diet derived sulforaphane. Mol Carcinog. 2015;55:2024–2036. doi: 10.1002/mc.22448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grun D, Adhikary G, Eckert RL. VEGF-A acts via neuropilin-1 to enhance epidermal cancer stem cell survival and formation of aggressive and highly vascularized tumors. Oncogene. 2016 doi: 10.1038/onc.2015.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zeng W, Chen X, Ma Y, Huang Z, Qin Y, Wu F, Wu L, Liang X, Qin Y, Zhou J, Lu D, Kuang X, Li QQ, Luo Z. A novel approach for enriching cancer stem cells from the human SW-13 adrenocortical carcinoma cell line. Anticancer Res. 2014;34:117–123. [PubMed] [Google Scholar]
- 33.Yu F, Yao H, Zhu P, Zhang X, Pan Q, Gong C, Huang Y, Hu X, Su F, Lieberman J, Song E. let-7 regulates self renewal and tumorigenicity of breast cancer cells. Cell. 2007;131:1109–1123. doi: 10.1016/j.cell.2007.10.054. [DOI] [PubMed] [Google Scholar]
- 34.Tan S, Chen JS, Sun LJ, Yao HR. Selective enrichment of hepatocellular cancer stem cells by chemotherapy. J Int Med Res. 2009;37:1046–1056. doi: 10.1177/147323000903700409. [DOI] [PubMed] [Google Scholar]
- 35.Boukamp P, Petrussevska RT, Breitkreutz D, Hornung J, Markham A, Fusenig NE. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J Cell Biol. 1988;106:761–771. doi: 10.1083/jcb.106.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dinkova-Kostova AT, Jenkins SN, Fahey JW, Ye L, Wehage SL, Liby KT, Stephenson KK, Wade KL, Talalay P. Protection against UV-light-induced skin carcinogenesis in SKH-1 high-risk mice by sulforaphane-containing broccoli sprout extracts. Cancer Lett. 2006;240:243–252. doi: 10.1016/j.canlet.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 37.Cheung KL, Kong AN. Molecular targets of dietary phenethyl isothiocyanate and sulforaphane for cancer chemoprevention. AAPS J. 2010;12:87–97. doi: 10.1208/s12248-009-9162-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gills JJ, Jeffery EH, Matusheski NV, Moon RC, Lantvit DD, Pezzuto JM. Sulforaphane prevents mouse skin tumorigenesis during the stage of promotion. Cancer Lett. 2006;236:72–79. doi: 10.1016/j.canlet.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 39.Boukamp P, Petrussevska RT, Breitkreutz D, Hornung J, Markham A, Fusenig NE. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J Cell Biol. 1988;106:761–771. doi: 10.1083/jcb.106.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Balasubramanian S, Adhikary G, Eckert RL. The Bmi-1 polycomb protein antagonizes the (−)-epigallocatechin-3-gallate-dependent suppression of skin cancer cell survival. Carcinogenesis. 2010;31:496–503. doi: 10.1093/carcin/bgp314. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 41.Singh SV, Herman-Antosiewicz A, Singh AV, Lew KL, Srivastava SK, Kamath R, Brown KD, Zhang L, Baskaran R. Sulforaphane-induced G2/M phase cell cycle arrest involves checkpoint kinase 2-mediated phosphorylation of cell division cycle 25C. J Biol Chem. 2004;279:25813–25822. doi: 10.1074/jbc.M313538200. [DOI] [PubMed] [Google Scholar]
- 42.Herman-Antosiewicz A, Xiao H, Lew KL, Singh SV. Induction of p21 protein protects against sulforaphane-induced mitotic arrest in LNCaP human prostate cancer cell line. Mol Cancer Ther. 2007;6:1673–1681. doi: 10.1158/1535-7163.MCT-06-0807. [DOI] [PubMed] [Google Scholar]
- 43.Siddik ZH. Cisplatin: mode of cytotoxic action and molecular basis of resistance. Oncogene. 2003;22:7265–7279. doi: 10.1038/sj.onc.1206933. [DOI] [PubMed] [Google Scholar]