Abstract
Introduction
Older adults with type 2 diabetes are at high risk of cognitive decline and dementia and form an important target group for dementia risk reduction studies. Despite evidence that computerized cognitive training (CCT) may benefit cognitive performance in cognitively healthy older adults and those with mild cognitive impairment, whether CCT may benefit cognitive performance or improve disease self-management in older diabetic adults has not been studied to date. In addition, whether adaptive difficulty levels and tailoring of interventions to individuals' cognitive profile are superior to generic training remains to be established.
Methods
Ninety community-dwelling older (age ≥ 65) diabetic adults are recruited and randomized into a tailored and adaptive computerized cognitive training condition or to a generic, nontailored, or adaptive CCT condition. Both groups complete an 8-week training program using the commercially available CogniFit program. The intervention is augmented by a range of behavior-change techniques, and participants in each condition are further randomized into a global or cognition-specific phone-based self-efficacy (SE) condition, or a no-SE condition. The primary outcome is global cognitive performance immediately after the intervention. Secondary outcomes include diabetes self-management, meta-memory, mood, and SE.
Discussion
This pilot study is the first trial evaluating the potential benefits of home-based tailored and adaptive CCT in relation to cognitive and disease self-management in older diabetic adults. Methodological strengths of this trial include the double-blind design, the clear identification of the proposed active ingredients of the intervention, and the use of evidence-based behavior-change techniques. Results from this study will indicate whether CCT has the potential to lower the risk of diabetes-related cognitive decline. The outcomes of the trial will also advance our understanding of essential intervention parameters required to improve or maintain cognitive function and enhance disease self-management in this at-risk group.
Keywords: Diabetes mellitus, Dementia, Mild cognitive impairment, Computerized cognitive training, Brain training, Self-management
1. Introduction
1.1. Diabetes is a risk factor for cognitive impairment and dementia
Dementia is firmly established as one of the most pressing public health concerns faced by societies worldwide, because of its very high and growing prevalence rates and the staggering direct and indirect costs associated with its management. Indeed, the World Health Organization ranked dementia third in terms of disease burden [1], and accordingly, it is listed as a National Health Priority in several countries, and coordinated global efforts to fight dementia are reflected in legislation (e.g., National Alzheimer's Project Act, 2011) and campaigns such as the National Plan to Address Alzheimer' Disease [2].
Several factors, including the lack of effective treatments to halt, alleviate, or reverse dementia symptoms, recent failures of phase 2–3 trials of disease-modifying treatments [3], the identification of modifiable risk and protective factors, and a prolonged preclinical phase, have contributed to the shifting of focus and resources to the possibility of preclinical prevention of dementia. Indeed, pharmacological and nonpharmacological intervention efforts increasingly target individuals at risk of dementia, reflecting hopes that interventions delivered before full-blown dementia develop are more likely to lead to improved outcomes.
Among the potentially modifiable risk factors for dementia, chronic metabolic conditions such as type-2 diabetes have been repeatedly shown to be associated with increased risk of cognitive decline [4], [5], conversion of mild cognitive impairment to dementia [6], and development of dementia-related disorders in general [7], [8], [9]. Although it has been suggested that midlife onset of diabetes is more strongly associated with dementia relative to onset of diabetes in older age [10], others found no modulating effect of diabetes duration on dementia risk [11], and yet others reported that relative to nondiabetic older adults, cognitive compromise in older diabetic adults is independent of age [12]. Among the overall number of worldwide cases of diabetes, which is currently estimated as 171 million and expected to increase to 366 million by the year 2030, type 2 is expected to represent most cases [13] and currently has a 12% to 25% prevalence rate among individuals aged 65 years and older [13], [14]. Therefore, the elderly population is slated to be most afflicted as the incidence of diabetes continues to climb, contributing to the risk of cognitive decline and dementia-related disorders in the elderly.
1.2. Cognitive impairment affects diabetes management
Importantly, even subtle decline in cognition and memory among people with diabetes has been shown to have negative implications on disease self-management [15]—the daily regimen that individuals with diabetes are expected to adhere to effectively manage their diabetes. Self-management in diabetes encompasses behaviors such as taking medication (orally and/or intravenously), monitoring blood glucose levels, exercising, adhering to appropriate dietary guidelines, foot care, and maintaining regular health care visits. In addition to the negative implications that compromised cognition has on diabetes self-management, untreated diabetes and poor self-management practice can themselves lead to progressively worse cognition [16]. Our group has previously reported that high hemoglobin A1c (HbA1c), which is a leading predictor of type 2 diabetes complications, modulates the association between prolonged untreated diabetes and cognitive functioning [17]. Furthermore, poor glucoregulatory control among untreated diabetic patients causes greater cognitive decrement [18], whereas improved glycemic control obtained by a reform of subsequent medication adherence can attenuate cognitive decline in individuals with diabetes [15], [19], [20]. Interestingly, in the ACCORD trial [21], a large randomized control trial (RCT) aimed at evaluating the effects of intensive pharmacological glycemic control in adults with type 2 diabetes, a small but significant benefit on the cognitive outcome (as reflected in performance on the Digit-Symbol Substitution Test) was found at the 20-month posttreatment evaluation in the intensive glycemic control relative to the standard treatment condition disappeared by an 80-month follow-up [22]. However, this finding could be explained by a range of factors, including that glycemic control in participants assigned to the intensive and standard treatment conditions at baseline was no longer different at the follow-up evaluation, reliance on a single test of processing speed to measure cognitive outcome, and participant dropout. Importantly, the intensive glycemic control condition was terminated prematurely due to increased mortality among participants in that arm, and the presence of more adverse events, including hypoglycemia and weight gain [21], highlighting the importance of careful lifestyle and risk factor management in the achievement of optimal disease control in type 2 diabetes.
1.3. Rationale for computerized cognitive training to improve cognition in diabetics
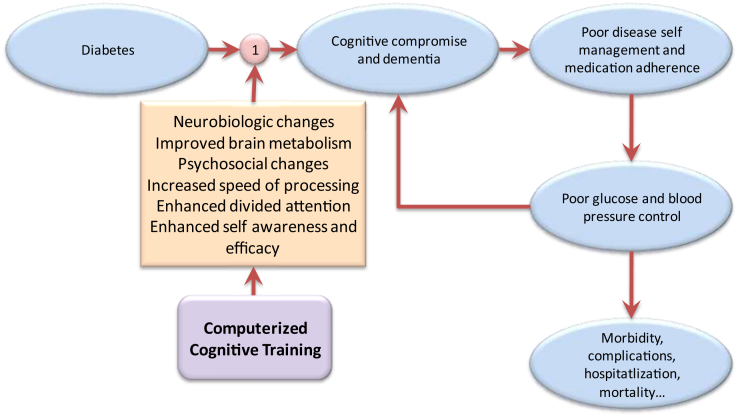
As effective diabetes self-management is central to minimizing the risk of complications, including cognitive and functional decline, and because of the possible contribution of preexisting, possibly subclinical cognitive impairment to ineffective self-management in diabetes, interventions to enhance cognitive functions have the potential to disrupt this downward spiral. A conceptual framework for cognitive training in diabetes is shown in Fig. 1. Although the evidence regarding the utility of cognitive training for persons with dementia is relatively negative [23], recent high-quality systematic reviews with meta-analysis encourage computerized cognitive training (CCT) in relation to cognitive outcomes in people at risk of dementia due to mild cognitive impairment (MCI) [24], [25], [26], as well as among cognitively healthy older adults [27], [28], [29]. However, whether CCT can enhance cognitive functioning, and importantly, contribute to improved disease self-management among older diabetic adults, has not been studied to date. Cognitive training in general, and CCT in particular, is appealing to users because such programs typically involve game-like tasks, so they are often experienced as fun and engaging. In addition, CCT programs can be readily completed from the comfort of one's home, which allows for widespread application. In recent years, adaptive task difficulty level as a function of performance and increasingly personalized task allocation are beginning to address the traditional criticism that CCT programs are delivered in a “one size fits all” approach. However, the extent to which these advances in CCT design lead to cognitive benefits over and beyond those gained from traditional CCT programs is yet to be determined and forms the basis for the choice of the control condition in the current trial. For researchers, CCT programs offer greater precision in measurement and automatic data collection and are therefore considered cost-effective. For these reasons, in recent years, CCT has largely replaced more traditional, pen-and-paper–based cognitive training.
Fig. 1.
A conceptual rationale for computerized cognitive training in relation to cognition and diabetes self-management in older diabetic adults.
The potential of cognitive training is supported by brain plasticity–related phenomena, confirming that the brain continuously changes at the molecular, synaptic, and cortical levels in response to experience, even in older age [30], [31]. Compared to intervention approaches that focus predominantly on compensatory mechanisms, such as cognitive rehabilitation [32], cognitive training assumes primarily restorative mechanisms of action, and that gains made in the context of training will transfer to untrained cognitive tasks as well as generalize to more distal areas of functioning [33], which is particularly pertinent in relation to improving disease self-management in older diabetic adults. The primary aim of the present study was therefore to evaluate, in older diabetic adults, the effects of a home-based personalized and adaptive CCT program on cognition and disease self-management, relative to an active control condition. It is hypothesized that personalized and adaptive CCT will be associated with greater gains in global cognitive performance and diabetes self-management relative to an active control condition immediately after the intervention and that these relative gains will be maintained at a six months' follow-up.
1.4. Behavioral strategies for improving intervention adherence
As with physical exercise programs and medication regimens, cognitive training programs are unlikely to yield significant benefit unless people adhere to them [34], yet managing adherence can be a challenge [35], especially in instances when the training is unsupervised [36]. Understanding and effectively targeting determinants of sustained health-related behavior change has been the subject of much research interest in recent years, as reflected in theoretical advances [37], [38] and the development of taxonomies of behavior-change techniques (BCTs) [39]. In particular, evidence suggests that self-efficacy (SE), the belief in one's potential to influence their situation through their actions [34], is important in relation to adherence to health-related behavior change interventions [40]. In published BCT taxonomies, specific techniques (BCTs) have been proposed as strategies to effectively boost SE, including vicarious experience, verbal persuasion, and focus on past success [39]. Systematically incorporating such techniques to any intervention, including CCT, may therefore arguably promote adherence. Whether SE is best viewed as a general or as a domain-specific characteristic of individuals (e.g., SE for physical activity, cognitive activity, etc.) is, however, not well established [40]. Hence, whether behavioral techniques used to boost SE in the context of efforts to optimize CCT adherence should target general SE, or be applied in a cognition-specific way, is not known. Accordingly, a secondary aim of the trial is to establish whether an SE intervention leads to improved adherence relative to receiving no SE intervention and whether differences exist between “general SE” and “cognition-specific SE” intervention.
2. Methods/design
2.1. Study setting and design
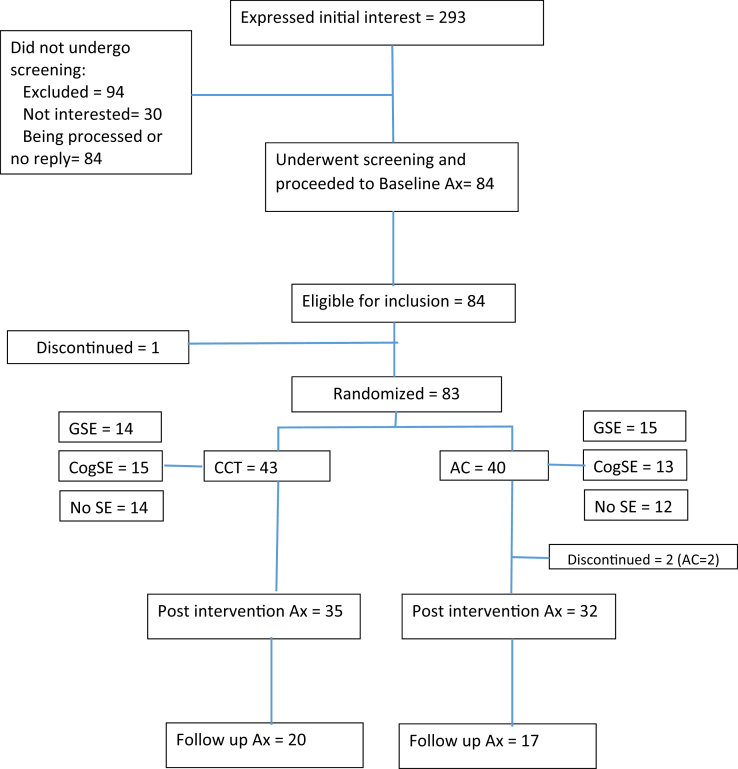
This is a protocol for the evaluation of an 8-week, home-based computerized cognitive training versus an active control intervention targeting nondemented diabetic older adults, in a double-blind RCT design. The study is conducted in the metropolitan Tel-Aviv area. Recruitment commenced in October 2015, and it is estimated that all follow-up assessments be completed by October 2017. The Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) Checklist is presented as Supplementary Material, and Fig. 2 is a SPIRIT-style flow chart of the trial.
Fig. 2.
Participant flow through the Sheba Medical Center computerized cognitive training trial (as of 1/03/17). Abbreviations: AC, active control; Ax, assessment; CCT, computerized cognitive training; cogSE, cognitive training–specific self-efficacy; GSE, global self-efficacy.
2.2. Participant eligibility and screening
Community-dwelling participants (n = 90) are recruited through advertising in the print and online media, through diabetes education groups, through fliers distributed in local health centers, through a large observational study (The Israel Diabetes and Cognitive Decline study) [41], and through word-of-mouth. Those expressing initial interest in the study by phone or e-mail are contacted to commence the screening process aimed at ascertaining whether inclusion/exclusion criteria are met (Table 1).
Table 1.
Trial inclusion and exclusion criteria
| Inclusion criteria | Exclusion criteria |
|---|---|
| Type 2 diabetes | Diagnosis of dementia/Alzheimer's disease |
| Fluency in Hebrew | Neurological or severe psychiatric disorder |
| Israeli resident living in the Tel-Aviv area | Commenced medication to improve mood or cognition within the last 3 months |
| Cover by Maccabi Health Services (MHS) | Significant visual or hearing impairment |
| Access to a computer and Internet at home | Compromised ability to perform day-to-day activities |
| Availability of a suitable informant | Involvement in a formal cognitive training intervention within the last 3 months |
| Formal education of 6+ years | Current participation in a clinical trial at Sheba Medical Center or MHS |
2.2.1. Sample size
Sample size calculations were estimated using G*Power (version 3.1.3) and were based on medium effect sizes found for cognitive outcomes in studies of cognitive training in cognitively healthy older adults and persons with MCI. In a recent trial targeting older adults with MCI, mood-related symptoms or both, and using the same CCT platform and control condition, we found moderate-to-large effects on a global cognitive composite [42]. For the present study, we made a conservative assumption of a small-medium effect size (F = 0.15) for group differences on the primary cognitive outcome (global cognitive performance). To detect a small-medium effect in a six-group design (2× training conditions, 3× SE conditions), with a 5% risk of type 1 error (α), 95% power, and an estimated correlation of r = 0.8 between repeated measurements of the primary cognitive outcome, a total sample size of 78 persons is required. Sample size calculations based on this six-group design will allow testing of the significance of the parameter estimates formed by the combination of assessment occasion, training condition, and SE group. The target sample size was set on 90, to account for an expected 15% dropout rate.
2.3. Interventions
Both the experimental and active control conditions involve repeated practice on standardized, game-like computer tasks, psychoeducation, and a range of BCTs used to optimize adherence and perseverance. Table 2 summarizes all intervention components included in the experimental and control conditions and the way in which these components were operationalized. Both groups train on a commercially available computerized cognitive training platform (CogniFit™), which has been used in several studies involving diverse populations [43], [44], [45], [46], [47]. The behavioral components of the interventions (as described in Table 2) were largely developed in reference to the taxonomy of BCTs and theoretical domains framework outlined by Michie et al. [37], [38].
Table 2.
Intervention components (including behavior-change techniques) included in the experimental and control conditions
| Intervention component/BCT | Component description | Adaptive training | Active control |
|---|---|---|---|
| Intervention introduction | |||
| Credible source | Information about possible health consequences (of diabetes) provided by a qualified professional (through the consent signing discussion) | Yes | Yes |
| Information about health consequences | Participants were provided with information about the possible consequences to their health of engaging in a “brain-training” intervention | Yes | Yes |
| Orientation to training environment∗ | Face-to-face orientation to the training platform and typical task requirements provided by trained staff | Yes | Yes |
| Training characteristics | |||
| Repeated practice on a selection of games/tasks∗ | CogniFit training games | Yes | Yes |
| Tasks/games designed to target specific cognitive domains∗ | CogniFit games were designed to reflect and target one or more cognitive domains | Yes | Yes |
| Adaptive task allocation∗ | The CogniFit games completed in each session were determined in an individually tailored way on the basis of the pretraining CogniFit cognitive assessment and were modified on the basis of ongoing online evaluation. | Yes | No |
| Adaptive task difficulty (variable challenge)∗ | The level of task difficulty changed as a function of performance within and across sessions | Yes | No |
| Task novelty∗ | Novelty of tasks/games varied such that new tasks/games are introduced more or less throughout the intervention period | Yes | Yes |
| Feedback on the outcomes of the behavior | Participants were given corrective feedback on their performance in the context of practice trials. Participants were also provided with feedback on their performance at the conclusion of a training session (i.e., session score). At the conclusion of the trial, participants were provided with written feedback about measures of cognition and well-being throughout the study phases. |
Yes Yes Yes |
Yes No Yes |
| Instruction on how to perform the behavior | Tasks/games included clear instructions and some practice to ensure instructions were understood. | Yes | Yes |
| Behavioral components | |||
| Goal setting (behavior) | Participants were encouraged to set the goal of training according to the prescribed dose (i.e., three days a week, between 20 and 30 minutes per session). | Yes | Yes |
| Commitment | It was recommended that participants form a commitment to train for the duration of the trial. (However, all were assured that they were free to withdraw from the trial at any stage without consequence.) | Yes | Yes |
| Schedule of contacts to maintain compliance | Participants were contacted by e-mail and phone using a fixed schedule to encourage compliance with the intervention and problem-solving barriers to participation. | Yes | Yes |
| Social support (practical) | Participants' spouse/close other was encouraged to provide ongoing practical support to facilitate participation in the intervention (e.g., quiet time), assistance with technical issues (with reminders from the study research person during fortnightly phone contact). | Yes | Yes |
| Restructuring the physical environment | Wherever possible, participants were asked to complete the training in a suitable and consistent environment (e.g., quiet room, free of distractions, at a similar time of the day, etc.) | Yes | Yes |
| Problem solving | During the orientation session, participants were encouraged to think about barriers to adherence to the intervention program (e.g., getting bored) and to reflect on possible solutions | Yes | Yes |
| Action planning | During the orientation session, participants were instructed to perform the behavior (i.e., training), 3 times per week, for approximately 30 minutes each time, over a period of 8–12 weeks. | Yes | Yes |
| Review behavior goals | During the fortnightly phone monitoring calls, participants progress with the training and any issues they were experiencing were discussed. Participants were given appropriate encouragement in relation to the behavioral training goals. | Yes | Yes |
| Behavioral contract | In all participant diaries, a written statement was included in which participants made a commitment to train at the prescribed dose. Participants signed this statement in the presence of the examiner. | Yes | Yes |
| Monitoring of the behavior by others without feedback | Participants were advised that the experimenters regularly monitor their training through the training web site. | Yes | Yes |
| Self-monitoring of behavior | Participants were instructed to record all training sessions in diaries given to them and were told the diaries will be collected on the end of training. | Yes | Yes |
| Self-monitoring of the outcomes of the behavior | Participants were provided with a cognitive score at the end of each training session and were also able to access a graph showing them their general progress throughout the training period. | Yes | No |
| Feedback on the outcomes of the behavior | Participants were provided with feedback regarding their neuropsychological test performance at the conclusion of the trial. | Yes | Yes |
| Past success† | During fortnightly telephone calls, participants were asked to recall a past success either relating to a training program they successfully completed or relating to a more general past success. | Yes | Yes |
| Verbal persuasion† | During fortnightly telephone calls, after learning of their personal past success (see above), participants were encouraged that they could successfully complete the current training as well. | Yes | Yes |
| Vicarious experience† | Participants were sent a video twice during their training of a peer that successfully completed the training. | Yes | Yes |
Abbreviation: BCT, behavior-change technique.
Intervention components not included in the published taxonomy of behavior change techniques (Michie et al. 2013).
BCTs used specifically as part of the self-efficacy (SE) intervention. These were delivered as either global or “domain-specific,” and participants in the “no-SE” group did not receive these components.
2.3.1. Group 1: Tailored and adaptive computerized cognitive training
On commencing the intervention, participants in the tailored and adaptive computerized cognitive training (TA-CCT) group complete a computerized cognitive assessment, included in the CogniFit platform, on the basis of which a profile of cognitive strengths and weaknesses is determined for each participant. Participants complete components of this assessment throughout their training period, such that their profile of cognitive strengths and weaknesses is continuously updated. Participants then go on to train on the 33 tasks that make up the CogniFit training platform (see Supplementary Table 1 for a list and a brief description of tasks), with task allocation being individually tailored by a computer algorithm, based on the cognitive profile established following the built-in assessment and updated throughout the training period. For example, a participant demonstrating weakness in the domain of spatial perception, but who scores high on measures of response time, is automatically allocated more tasks intended to improve spatial perception and fewer tasks intended to improve response time. Note that the CogniFit-delivered cognitive assessment at the start and end of training is distinct from the neurocognitive assessments conducted as part of the study outcome evaluations described in the following. In addition, the difficulty level of tasks is adaptive to participants in this group within and across sessions, such that the level of challenge always reflects individual performance. At the conclusion of each training session, participants in this group are presented with their session score, and participants are therefore provided with feedback on their performance and can track their progress.
2.3.2. Group 2: Active control group
In keeping with our findings from a recent trial of CCT in MCI [42], participants in the active control (AC) condition complete a training program that is identical to the one completed by participants in the TA-CCT condition in all but three features: (1) No individual tailoring. Task allocation for participants in this group is not based on their personal cognitive profile but is instead generic, and task distribution to all participants is similar. (2) No adaptive difficulty level. Although within a session a given task becomes harder with better performance, the level of task difficulty across sessions is uniform. (3) Participants in this group view their baseline and end-of-training score on the built-in computerized evaluation; however, they do not receive session-based performance feedback.
In all other respects, the two training groups are indistinguishable, allowing for blinding of participants to group membership. Participants in both groups are instructed to train three times per week, preferably alternating between training and rest days. On each training day, participants are asked to complete two training sessions, lasting 10–15 minutes each (for a total of 20–30 minutes per training day, and six training sessions per week) for the duration of 8 weeks (total of 48 sessions). The prescribed intervention intensity is in keeping with our own experience [48], and that of others [49], and supported by the findings from a recent meta-analysis [29]. Based on our recent findings [42], an 8-week duration was deemed sufficient to detect group differences on the primary outcome. Each training session includes a unique combination of three types of tasks reflecting a range of cognitive abilities. The wide variety of tasks is intended to encompass a broad range of cognitive functions and serves also to keep the participant interested and engaged. Participants in both groups complete identical orientation sessions to the training environment (during which participants complete the baseline computerized cognitive evaluation and receive a printed training manual and a diary). Finally, participants were all contacted over the phone every 2 weeks to provide technical assistance as required, inquire about their mood, and provide general support, using a scripted protocol.
2.4. SE interventions
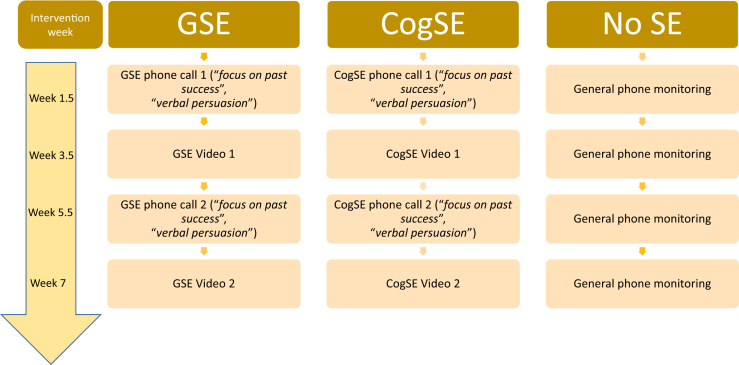
As can be seen in Table 2, three BCTs were included to specifically target SE (“focus on past success,” “verbal persuasion,” and “vicarious experience”), and these are delivered in the context of fully scripted monitoring phone calls and augmented by short video clips sent to participants, as further described in the following and summarized in Fig. 3 (materials available from the authors on request). During the monitoring phone call for the global SE condition, the script is worded in a general way (e.g., “can you tell me of a time in the past in which you succeeded in achieving a goal that involved perseverance or adherence to a program?”), whereas in the cognition-specific SE condition, the script is worded specifically in relation to a cognitively challenging activity (e.g., “Can you tell me of a time in the past in which you succeeded in achieving a goal that involved perseverance or adherence to a mentally challenging goal?”). During these phone calls, participants in both SE conditions are given the opportunity to discuss their past experiences and are guided to focus on either a general or a cognitive-specific past experience, depending on their condition allocation. Furthermore, two videos in which an actor relays their account of a general past success are sent to participants in the global SE condition during the course of the intervention (video #1 of a male actor speaking about his professional success as a result of perseverance and video #2 of a female actor speaking about achieved weight loss due to commitment and hard work). Participants in the cognition-specific SE condition are also sent two videos in which the actor (video #1 male actor and video # 2 female actor) relays their success in a CCT intervention. The phone-based and video-based delivery of SE intervention, through which participants were exposed to the relevant BCTs, ensured that the SE support was multimodal, consistent with evidence suggesting that a multisource approach to SE intervention is more effective [50]. Finally, participants allocated to the “no-SE” condition received the standard phone monitoring call every 2 weeks, but these calls did not include the delivery of SE-specific BCTs, and no videos were sent to these participants.
Fig. 3.
Schedule and format of delivery of relevant Behaviour Change Techniques in the self-efficacy conditions. Abbreviations: cogSE, cognitive training–specific self-efficacy; GSE, global self-efficacy; SE, self-efficacy.
Adherence and compliance to the program protocol is further monitored in two ways: (1) Automatic records: CogniFit maintains an electronic log of all training data, including time of login and log out, tasks completed, time spent on the overall session and on each task, and performance indicators (e.g., reaction time, error rate, etc.). (2) Personal diaries: each participant receives, on training commencement, a printed diary (available from the authors) with weekly templates into which they are asked to enter details regarding their training. This strategy provides participants with an opportunity for self-monitoring of their own progress. The automatically recorded session logs are the primary method for determining adherence for the purpose of data analysis. Over the phone (and if required, at home), technical assistance is available to participants in both groups daily. Study participants are advised that throughout the trial period (including the nonintervention periods), they are not permitted to take part in any other clinical trial or systematic cognitive intervention, although they are encouraged to continue engaging with any cognitively challenging activities forming part of their routine.
2.4.1. Booster training
Previous studies indicate that booster training may contribute to the maintenance of CCT-related training gains (e.g., [51]). Accordingly, 3 months after the completion of the main training phase, participants are invited to log back to their training accounts and complete three more training sessions over a 1-week period. Participants receive only one invitation to complete the booster training sessions, and no further prompting is provided. Access to the training platform is blocked 2 weeks after the invitation to complete booster training is sent.
2.5. Randomization and masking procedure
After the baseline assessment and confirmation of eligibility, participants are allocated to the TA-CCT (n = 45) or the AC (n = 45) conditions using a block-randomization procedure. Within each training condition, participants are further randomized into a global SE (GSE, n = 15), a cognition-specific SE (CogSE, n = 15), or a no-SE condition (no-SE, n = 15). Randomization sequences are produced by a computer (www.randomizer.org) and are concealed from study personnel by a researcher unrelated to the study, who provides the group allocation for each participant on request from the trial manager. Participants are unaware of their group allocation and are told that they are allocated to one of two versions of the training program and to one of three “encouragement” groups which differ in some aspects. Participants are not told of any specific hypothesis associated with any of the training versions and are therefore unlikely to be able to guess their group allocation. In a brief online post-study survey, participants are asked to respond to a question assessing their belief regarding whether they completed the experimental or the control intervention, which will serve as a measure of the success of the masking procedure. Research staff completing the postintervention and follow-up assessments are blind to intervention and SE conditions.
2.6. Assessments, outcomes, and measures
Participants are assessed on inclusion in the study (baseline), as well as immediately after the intervention (postintervention) and at 6-month follow-up. All assessments are conducted by psychologists trained in the administration and scoring of neurocognitive tests, under the supervision and with regular monitoring of one of the trial managers (A.B.-F.). A complete list of measures used in the study to measure the cognitive and noncognitive outcomes is shown in Table 3. The primary outcome is change in general cognitive ability, as reflected in a composite global cognitive score derived from the cognitive assessment battery, at the end of the 8-week intervention. The pen-and-pencil battery, administered in face-to-face sessions, includes commonly used cognitive measures selected to reflect a range of cognitive domains such as attention, executive functions, language processing, learning, and memory. As noted previously, the cognitive assessment is independent from the computerized cognitive evaluation completed as part of training by CogniFit.
Table 3.
Details of the assessment battery and associated measures
| Cognitive measures | ||||
|---|---|---|---|---|
| Test name | Primary domains | Subtests administered/measures derived | Reference | Source of norms/cutoffs |
| M-ACE | Attention, Memory, Fluency, Memory, Language, Visuospatial Abilities | N/A | Hsieh, 2015 | Hsieh, 2015 |
| L'Hermitte Board | Visuospatial working memory and learning | Learning Trials†, Delayed Recall∗ | L'hermitte and Signoret, 1972 | Normative data for older Australian |
| Logical Memory | Verbal attention, Verbal Short Term Memory | Immediate Recall†, Delayed Recall∗ | Wechsler, 1997 | IDCD baseline |
| RAVLT | Auditory Attention, Verbal Learning, Strategy Formation, Verbal Short Term Memory | Learning Trials†, Short Term Recall†, Delayed Recall∗, Recognition† | Strauss, Sherman and Spreen, 2006 | Vakil, 1997, Vakil, 2010 & normative data for Australian norms |
| ROCFT | Visual Perception, Visuoconstruction, Planning, Organization, Visuospatial Memory | Copy‡, Immediate Recall†, Delayed Recall∗, Recognition† | Osterrieth, 1944 | Normative data for Australian norms |
| Verbal Fluency | Phonemic Fluency, Semantic Fluency, Attention Switching | Letter Fluency (FAS)‡, Category Fluency (animals, boys names)‡, Category Switching (fruit and furniture)‡ | Spreen & Strauss, 1998 | IDCD baseline, AIBL baseline HC and BBL baseline |
| Digit Span | Immediate Auditory Attention Span, Auditory Working Memory | Digit-Span Forwards‡, Digit-Span Backwards‡ | Wechsler, 1997 | AIBL baseline HC and IDCD baseline |
| Digit-Symbol Coding | Processing Speed, Visual Working Memory | Coding‡ | Wechsler, 1997 | IDCD baseline |
| Boston Naming test (15 items) | Semantic Memory | Naming visual items‡ | Kaplan, Goodglass & Weintraub, 1987 | IDCD baseline |
| Trail-Making Task |
Processing Speed, Visual Search, Attention Switching |
Trail A‡, Trail B‡ |
Reitan & Wolfson, 1993 |
IDCD baseline |
| Questionnaires |
||||
| Scale name |
Subsets/domains administered |
Respondent |
Reference |
|
| MMQ | Contentment, Memory Mistakes/Ability, Memory Strategies | Participant | Troyer et al., 2002 | N/A |
| CDR | Global dementia rating, sum of boxes | Informant/clinician | Morris, 1993 | Morris, 1993 |
| GDS | Mood—Depression | Participant | Sheikh & Yesavage, 1986 | Burke et al., 1991 |
| GAI | Mood—Anxiety | Participant | Pachana, 2007 | Pachana, 2007 |
| DSMQ | Diabetes self-management | Participant and informant | Schmitt, 2013 | N/A |
| GSES | General self-efficacy | Participant | Zeidner et al., 1993 | N/A |
| CT-SES | Self-efficacy for cognitive training | Participant | Not published | N/A |
| NPI-Q | Mood—neuropsychiatric symptoms | Informant | Cummings et al., 1994 | N/A |
| ZBI | Caregiving burden | Informant | Zarit, Reever & Back-Peterson, 1980 | N/A |
| BADL | Activities of daily living—functional independence | Informant | Bucks et al., 1996 | Bucks et al., 1996 |
Abbreviations: BADL, Bristol Activities of Daily Living; CDR, Clinical Dementia Rating; CT-SES, Cognitive-training Self-Efficacy Scale; DSMQ, Diabetes Self-Management Questionnaire; GAI, Geriatric Anxiety Inventory; GDS, Geriatric Depression Scale; GSES, Generalized Self-Efficacy Scale; IDCD, Israel Diabetes and Cognitive Decline study; M-ACE, Mini-Addenbrooke Cognitive Evaluation; MMQ, Meta Memory Questionnaire; NPI-Q, Neuropsychiatric Inventory Questionnaire; RAVLT, Rey Auditory Verbal Learning Test; ROFCT, Rey Osterrieth Figure Copy Test; ZBI, Zarit Burden Interview.
NOTE. A total score for each test was created by averaging the Z scores from all the indices that make up that test. The total Z scores from all cognitive tests were then averaged to create the composite global cognitive outcome. IDCD Baseline: Guerrero-Berroa et al., 2014; AIBL Baseline: Ellis et al., 2009; BBL Baseline: Anstey et al., 2015.
Score included in delayed memory composite.
Score included in learning and memory composite.
Score included in composite nonmemory.
Change in the following secondary outcomes will also be assessed after intervention and at the 6-month follow-up assessment.
2.6.1. Patient outcomes
-
1.
Diabetes self-management. Diabetes self-management is measured with a self and informant version of the Diabetes Self-Management Questionnaire [52], a 16-item questionnaire assessing diabetes-related self-care activities. The questionnaire includes four subscales: “Glucose Management,” “Dietary Control,” “Physical Activity,” and “Health-Care Use,” as well as a “Sum Scale” (max score = 10) as a global measure of self-care. Response options range from 0 (never occurs) to 4 (occurs very frequently). The total score of the Diabetes Self-Management Questionnaire was found to have adequate internal consistency (Cronbach's α coefficient = 0.84), and the subscales were found to have acceptable consistencies (α coefficients = 0.77 for “Glucose Management,” 0.77 for “Dietary Control,” 0.76 for “Physical Activity,” and 0.60 for “Health-Care Use”). This outcome will be assessed separately for the self- and the informant-rated versions of this scale.
-
2.
Generalized SE, as measured by the Generalized Self-Efficacy Scale [53], a 13-item scale assessing a general sense of perceived SE. Responses are made on a four-point scale and are summed to yield a total score.
-
3.
Cognitive training–related SE, as measured by the Cognitive-Training Self-Efficacy Scale, a nine-item scale developed for the present study, and modeled on a published domain-specific SE scale—the Self-Efficacy for Diabetes Self-Management Scale [54]. Participants are asked to rate the extent to which they feel confident they can successfully complete an assigned cognitive training task across various situations (e.g., when tired, bored, stressed, etc.). Responses are made on a five-point scale (from “very confident” to “not confident at all”), and all nine responses are summed to yield a total score (max = 45)
-
4.
Everyday memory functioning, as measured by the Meta Memory Questionnaire (MMQ) [55]. The MMQ is a self-rated memory questionnaire that includes three subscales: Contentment (i.e., affect regarding one's memory), Ability (i.e., self-appraisal of one's memory capabilities), and Strategy (i.e., reported frequency of memory strategy-use). Each of the MMQ subscales yields a total score, which will be evaluated separately after the intervention.
-
5.
Mood-related symptoms, as measured by a composite mood score derived from the self-reported Geriatric Depression Scale short-form 15 [56], and the Geriatric Anxiety Inventory [57]. Additional analyses will be conducted for each mood domain (anxiety, depression) separately.
-
6.
Activities of daily living as measured by the Bristol Activities of Daily Living Scale (BADL) [58], a 20-item informant-rated measure of functional capacity designed to quantify functional disability and change in function among persons with dementia. The scale covers four domains (self-care, mobility, orientation, and instrumental ADLs), and higher scores (max = 60) reflect greater functional disability. The scale is administered as part of the screening procedure to exclude participants showing signs of functional decline (BADL < 8), as well as in subsequent assessments to measure change in function.
-
7.
Dementia severity, as measured by the Clinical Dementia Rating Scale (CDR) [59]. The CDR is administered at study entry to exclude participants with dementia (CDR ≥ 1), and at the 6-month follow-up to evaluate dementia severity (CDR total) and functional status (Sum of Boxes score).
2.6.2. Informant outcomes
-
8.
Caregiving burden, as measured by scores on the Zarit Burden Interview [60], a 22-item informant-rated scale evaluating the impact of the participant's disabilities on their life. Originally designed to evaluate burden associated with caregiving for dementia patients, the Zarit Burden Interview has since been used to evaluate disability-related burden associated with other age-related conditions [51], [52].
-
9.
Caregiver distress as measured by scores on the distress subscale of the Neuropsychiatric Inventory Questionnaire (NPI-Q) [61]. The NPI-Q is an informant-reported questionnaire based on the original NPI [62], measuring the presence and severity of symptoms in 13 categories of neuropsychiatric behavioral disturbance, along with the distress felt by the informant. The total severity score is the sum of the severity scores obtained for each behavioral category.
In addition, participants in the present study also gave consent for the research team to obtain relevant demographic and diabetes-related data (e.g., years since diagnosis, types of medications, HbA1C levels, medications prescribed, etc.) from the diabetes registry maintained by Maccabi Health Services from where all participants were recruited. Relevant data will be obtained to conduct additional secondary exploratory analyses of candidate predictors of treatment response to facilitate optimal design of a large-scale CCT trial, if it is found beneficial.
2.7. Procedure
Individuals expressing interest in the study are sent general information about the study by e-mail, including a short self-assessment questionnaire, which serves to screen for individuals that do not meet the study's eligibility criteria. Participants meeting the basic eligibility (Table 1) are invited to proceed to the second screening phase, as part of which the BADL is administered to the nominated informant during a short phone interview to verify functional independence. Participants are then invited to attend an information session at the Sheba Medical Center, and those interested are invited to sign the consent form. The details of participants who consent to participate are then passed to the researcher responsible for conducting the baseline evaluation, who arranges the usually home-based baseline assessment. On completion of the baseline evaluation, participants are randomized into the training (TA-CCT or AC) and SE (GSE, CogSE, No-SE) conditions as described previously, and their details are passed to the research person responsible for orienting them into the intervention. During the orientation to the intervention (completed according to a structured and scripted protocol), a step-by-step review of a written user manual is completed, along with instruction on completion of the participant intervention diary, completion of the baseline computerized assessment included in the CogniFit training platform, and completion of a general SE scale, and an SE scale for cognitive training (see Table 2).
During the intervention period, participants receive a fortnightly phone call aimed at monitoring the progress of participants through the training, their mood, and well-being and providing technical support as required. In addition, depending on the group to which a participant is assigned, the SE intervention is also delivered during this phone call, as described previously. The structure and content of these phone calls are fully scripted, and fidelity of delivery is evaluated on an ongoing basis with the trial manager.
At the end of the 8-week intervention period, participants are invited by e-mail to complete again the computerized cognitive evaluation included in the CogniFit platform. At this point, their details are passed again to the blind assessors, who scheduled the postintervention pen-and-paper assessment session, typically within 1 to 2 weeks of completion of the training. Three months after the completion of the training phase, participants are contacted by e-mail and invited to complete the 1-week booster training, during which they are again asked to complete six 20-minute training sessions on CogniFit. Finally, 6 months after the end of the training phase, participants are invited to undergo the final cognitive evaluation, which is identical to the previous assessment sessions.
2.8. Noncompliance and attrition
As noted previously, adherence to the intervention protocol is monitored automatically through CogniFit's log files. Participants are considered to have been compliant with the training if they completed at least 80% of the total training dose (38 training sessions). Nonadherence does not lead, however, to the exclusion of participants from the trial, and participants who do not adhere to the intervention protocol or advised that they wish to discontinue the intervention are invited to undergo the postintervention and follow-up assessments at the usual times.
2.9. Data management
A comprehensive, password-protected file is used to maintain a registry of all expressions of interest, as well as all data relevant to the screening procedures and to manage the schedule for relevant events throughout the various stages of the trial. All consent forms, signed by the participant and cosigned by one of the principal investigators, are stored separately from deidentified assessment and intervention-related data, consistent with the requirements of the hospital's ethics committee on completion and scoring of all pen-and-paper assessments, scores are entered into a hard copy and electronic client record form. Both pen-and-paper assessment protocols and CRFs are stored in locked cabinets and password-protected files, accessible only by the research team. Assessment data included in the computerized cognitive evaluations conducted by the CogniFit platform at baseline and continuously throughout the training are downloaded as Excel spreadsheets directly from CogniFit's servers on completion of the training. The CRFs of all participants are reviewed for accuracy by the trial manager. The trial was selected for an audit by the Sheba Medical Centre's Division for Research and Development Committee which subsequently reviewed all relevant procedures, including consent-related procedures and data management (18 April 2016), and provided a favorable review of the trial's procedures.
2.10. Statistical analysis
Data analyses will commence once the last participant completes their final assessment and will be conducted using IBM SPSS Statistics (V.22) and Stata (V.13; StataCorp, Texas, USA). All data will be first scrutinized for completeness, distributions, the presence of outliers, and missing data. Missing data will be handled in accordance with the steps recommended by Carpenter and Kenward [63], namely analyses of outcome variables with less than 15% of the data deemed to be missing completely at random (MCAR) will be proceeded using observed data only. This is not considered a significant issue for linear mixed models as these techniques include cases with missing data. Data will be considered to be MCAR when no associations will be found between the values on variables with missing data and other observed data, and Little's chi square test, included in SPSS missing data analysis, will be used to evaluate the null hypothesis that data are MCAR. In the event that data will found to be missing at random, missing values will be imputed using multiple regression models, and if the data are deemed to be not missing at random, the SPSS EM procedure will be used to estimate means and standard deviations on relevant variables while taking into account the pattern of missing data. Distributions showing substantial deviations from normality will be transformed to reduce the influence of extreme values. All cognitive test scores are standardized against local norms where available or to published international norms where local norms are not available (see Table 2). In addition, when more than one score was derived from a test (e.g., immediate and delayed recall measures), a total score for that test is derived by computing the average Z score of all indices from that test. The Global Cognitive score is the mean of the Z scores of all cognitive measures. A composite Delayed Memory score is computed as the mean Z score of the delayed recall trials of the Logical Memory, RAVLT, and ROFCT tests. A composite Learning and Memory score is computed as the mean Z score of the total score of each memory test. Finally, a Non-Memory composite score is calculated from the following measures: RCFT (copy); phonetic, semantic, and switching fluency; digit-symbol coding; digit-span; and trail-making A+B. Scores on the self-reported mood scales (Geriatric Depression Scale and Geriatric Anxiety Inventory) are also converted into Z scores against the baseline mean and standard deviation of the complete sample.
All analyses will be completed using an intention-to-treat approach. Baseline characteristics of the two intervention conditions will be compared using Analysis of Variance for continuous variables and chi-square for categorical variables. Because of the typical dropout rates in clinical trials, and to the fact that central assumptions of the general linear model (uncorrelated observations, homogeneity of variance) are typically not met in the context of repeated measurements in a clinical trial, linear mixed models will be used to model the association between predictors and each of primary and secondary outcome variables. The following specifications will be used in all fitted models.
In relation to each outcome measure of interest, the fit statistics Bayesian Information Criterion of three primary models will be compared. The basic model will include fixed main effects of training condition (TA-CCT vs. AC; reference condition), SE condition (No-SE; reference, CogSE, GSE), and we will compare basic models in which assessment occasion will be specified as a fixed effect (T0; reference time, T1, T2), with models in which it will be specified as having a random slope. The second model will include the aforementioned terms (with assessment occasion as a fixed effect) as well as the two-way interactions (between time and training condition, time and SE condition, and training condition and SE condition). Finally, the third model will include all the above, as well as the three-way interaction between assessment occasion, training condition, and SE condition. Participants will be specified as having a random slope in all models. Model parameters will be estimated using Restricted Maximum Likelihood Method, with an Unstructured Covariance Matrix specified to model the covariance of the residuals and the random factors. Based on the rule of thumb provided by Seltman [64], a reduction of greater than two points in the Bayesian Information Criterion will generally be considered a relative improvement in the model and used as the basis for model selection. Results will be considered to be statistically significant when P ≤ .05.
For each of the primary and secondary outcomes, effect sizes will be calculated as the standardized group differences in change scores between baseline and postintervention assessment, as well as between baseline and follow-up assessments (Cohen's d).
3. Discussion
The development of effective dementia risk reduction interventions is a public health priority, and individuals with diabetes represent an important target group for such interventions due to their consistently demonstrated increased risk. Ideally, such interventions should be embedded in lifestyle choices, be motivating and engaging, and be readily scalable to wide sections of the population. CCT satisfies many of the requirements of effective public health interventions and in recent years has indeed become a popular approach in research settings as well as in the wider population.
To our knowledge, this is the first RCT of CCT targeting cognition and disease self-management among older diabetic adults. While based on previous studies we expect that benefits in cognitive performance will be observed, the present study will further clarify whether personalization of task allocation algorithms, adaptive difficulty levels, and the provision of feedback are critical intervention components over and beyond training on the same tasks but without these intervention features. The study will further clarify whether CCT has an impact on disease self-management and the extent of the association between cognitive function and disease self-management. Furthermore, the study will advance our understanding of the role of global and cognition-specific SE in relation to intervention adherence and study outcomes. Taken together, data from the current trial will inform future efforts to optimize cognition and disease self-management in this population, which may play a role in the prevention of dementia. The protocol in this study can additionally be implemented in the study of different populations that are at risk for cognitive impairment and in which this impairment is associated with poor disease self-management.
Research in Context.
-
1.
Systematic review: Numerous studies have pointed out the potential of computerized cognitive training interventions in improving cognitive outcomes in cognitively healthy older adults, and those with mild cognitive impairment, but whether such interventions are associated with cognitive benefits and improved self-management in older adults at risk of dementia due to diabetes is unknown.
-
2.
Interpretation: Once completed, the intervention described in this protocol will clarify whether computerized cognitive training plays a role in the self-management of older diabetic adults, potentially interfering with a downward spiral resulting from the interplay between subtle cognitive impairment and suboptimal diabetes self-management in older adults.
-
3.
Future directions: The findings from the trial described in the current protocol will have implications for the design of future cognitive training interventions to maximize their potential to not only improve or sustain cognitive functioning but also promote effective self-management in lifestyle and age-related chronic conditions associated with increased risk of dementia.
Acknowledgments
The authors wish to thank the participants who took part in this study and their helpful informants. The authors further wish to thank Yonatan Shwartz, Or Kirshenboim, Amir Cohen, and Mirit Luzon for their assistance with participant assessments and Itzik Cooper for assistance with randomization.
This study was conducted with the support of an MHS grant to Michal Schnaider-Beeri (grant no. 25860). The funding source played no role in the design and implementation of the trial, analysis and interpretation of the data, or preparation of the article. The CCT platform was donated by CogniFit™. CogniFit or its employees played no role in the design and implementation of the trial, analysis and interpretation of the data, or preparation of the article. Rachel Bloom is supported by the Vice-Chancellor Award awarded to her by Bar Ilan University. Alex Bahar-Fuchs is supported by an Australian National Health and Medical Research Council fellowship (grant no. 1072688).
Authors' contributions: A.B.-F. conceived and designed the trial and managed all aspects of implementation of the trial, provided training and supervision to the trial team, and contributed to the writing and critical review of the article. M.S.-B. conceived and designed the trial, provided overall supervision, and contributed to the drafting and revision of the article. R.B. conceived and designed the SE intervention delivered as part of the trial, coordinated and contributed to aspects of the data collection, and wrote the article. R.R. contributed to the study design, oversaw ethical considerations, and made significant contribution to the drafting and revision of the article. Y.R. contributed to the study design and made significant contribution to the drafting and revision of the article. A.H. contributed to the study design and made significant contribution to the drafting and revision of the article. H.D. contributed to the data collection, contributed to the delivery of the interventions, and provided comments on drafts of the article. L.B. contributed to the data collection, contributed to the delivery of the interventions, and provided comments on drafts of the article. S.S. contributed to the data collection, contributed to the delivery of the interventions, and provided comments on drafts of the article. All authors have given approval for the final version of the article.
The data set generated and analyzed during this study is not publicly available at this point as the trial is still under way. The data set will be made available to authors in the future on reasonable request. Detailed assessment and intervention protocols are available and will be provided to authors on reasonable request from the corresponding author.
The trial was approved by the Ethical Review Boards at Sheba Medical Center (SMC-0573-13) and at Maccabi Health Services (MHS 25/2014). All participants in the study are functionally independent and either cognitively healthy or showing signs of mild cognitive impairment, and therefore all considered competent to give informed consent. Before signing consent, all participants are invited to attend an information session in which all relevant details are provided. At the conclusion of each information session and before signing the informed consent, participants are given a brief questionnaire evaluating their understanding of main features and procedures in the study. In the event that responses to any of the questions are incorrect, the trial manager further discussed the relevant issues with the participant to ensure understanding before allowing the participant to sign the consent form. Written consent was obtained by trial manager in all cases. Based on prior studies in this area, participants in both the experimental and control conditions are expected to derive some benefit from the intervention. Equally, based on past research, the interventions are expected to lead to no or minimal and infrequent adverse events, limited to transient training-related emotional states of anxiety and depression. Whether such adverse events occurred was evaluated as part of the fortnightly monitoring phone calls and managed in accordance with a written protocol to ensure the well-being of all participants. In the unlikely event that a participant develops an adverse psychological reaction associated with study participation, and following a consensus decision of a monitoring committee comprised an experienced geriatric psychiatrist (R.R.-S.) and the trial manager (A.B.-F.), they will be excluded from the study and referred to their primary physician for further management. Participants are advised that, in the event that one of the two intervention conditions turns out to be more effective than the other in relation to the primary cognitive outcome, those assigned to the less effective treatment condition will receive access to the more effective treatment condition for up to 12 months. Participants are further advised that they will receive a brief, simple language summary of the trial results by e-mail at the conclusion of statistical analyses and that the summary of findings will be posted on the web site of the Joseph Sagol Neuroscience Center as well. Personally identifiable information (including consent forms and electronic participant register) is saved separately from deidentified research data and is managed in accordance with the standards set by the Israeli Health Ministry, and consistent with Good Clinical Practice guidelines. All research personnel are required to hold a current Good Clinical Practice certificate from a Sheba Medical Center–approved provider and to sign a confidentiality statement.
Footnotes
Trial registration: www.ClinicalTrials.Gov, NCT02709629, retrospectively registered on: 25/2/16.
A.H is an employee of MHS who provided funding for this study. The authors declare that they have no competing interests.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.trci.2017.10.003.
Supplementary data
References
- 1.World Health Organization . World Health Organization; Geneva: 2010. mhGAP Intervention Guide for Mental, Neurological and Substance Use Disorders in Non-specialized Health settings: Mental Health Gap Action Programme (mhGAP) [PubMed] [Google Scholar]
- 2.US Department of Health and Human Services . Assistant Secretary for Planning and Evaluation (ASPE); Washington, D.C: 2016. National Plan to Address Alzheimer's Disease: 2016 Update. [Google Scholar]
- 3.Cummings J., Morstorf T., Lee G. Alzheimer's drug-development pipeline: 2016. Alzheimers Dement (N Y) 2016;2:222–232. doi: 10.1016/j.trci.2016.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Monette M.C., Baird A., Jackson D.L. A meta-analysis of cognitive functioning in nondemented adults with type 2 diabetes mellitus. Can J Diabetes. 2014;38:401–408. doi: 10.1016/j.jcjd.2014.01.014. [DOI] [PubMed] [Google Scholar]
- 5.Palta P., Schneider A.L., Biessels G.J., Touradji P., Hill-Briggs F. Magnitude of cognitive dysfunction in adults with type 2 diabetes: a meta-analysis of six cognitive domains and the most frequently reported neuropsychological tests within domains. J Int Neuropsychol Soc. 2014;20:278–291. doi: 10.1017/S1355617713001483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cooper C., Sommerlad A., Lyketsos C.G., Livingston G. Modifiable predictors of dementia in mild cognitive impairment: a systematic review and meta-analysis. Am J Psychiatry. 2015;172:323–334. doi: 10.1176/appi.ajp.2014.14070878. [DOI] [PubMed] [Google Scholar]
- 7.Deckers K., van Boxtel M.P., Schiepers O.J., de Vugt M., Muñoz Sánchez J.L., Anstey K.J. Target risk factors for dementia prevention: a systematic review and Delphi consensus study on the evidence from observational studies. Int J Geriatr Psychiatry. 2015;30:234–246. doi: 10.1002/gps.4245. [DOI] [PubMed] [Google Scholar]
- 8.Ravona-Springer R., Schnaider-Beeri M., Goldbourt U. Body weight variability in midlife and risk for dementia in old age. Neurology. 2013;80:1677–1683. doi: 10.1212/WNL.0b013e3182904cee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu W., Tan L., Wang H.F., Jiang T., Tan M.S., Tan L. Meta-analysis of modifiable risk factors for Alzheimer's disease. J Neurol Neurosurg Psychiatry. 2015;86:1299–1306. doi: 10.1136/jnnp-2015-310548. [DOI] [PubMed] [Google Scholar]
- 10.Xu W., Qiu C., Gatz M., Pedersen N.L., Johansson B., Fratiglioni L. Mid-and late-life diabetes in relation to the risk of dementia. Diabetes. 2009;58:71–77. doi: 10.2337/db08-0586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leibson C.L., Rocca W.A., Hanson V.A., Cha R., Kokmen E., O'Brien P.C. Risk of dementia among persons with diabetes mellitus: a population-based cohort study. Am J Epidemiol. 1997;145:301–308. doi: 10.1093/oxfordjournals.aje.a009106. [DOI] [PubMed] [Google Scholar]
- 12.Yeung S.E., Fischer A.L., Dixon R.A. Exploring effects of type 2 diabetes on cognitive functioning in older adults. Neuropsychology. 2009;23:1. doi: 10.1037/a0013849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rathmann W., Giani G. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:2568–2569. doi: 10.2337/diacare.27.10.2568. [DOI] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention . US Department of Health and Human Services; Atlanta, GA: 2014. National Diabetes Statistics Report: Estimates of Diabetes and its Burden in the United States, 2014. [Google Scholar]
- 15.Grodstein F., Chen J., Wilson R.S., Manson J.E. Type 2 diabetes and cognitive function in community-dwelling elderly women. Diabetes Care. 2001;24:1060–1065. doi: 10.2337/diacare.24.6.1060. [DOI] [PubMed] [Google Scholar]
- 16.Feil D.G., Zhu C.W., Sultzer D.L. The relationship between cognitive impairment and diabetes self-management in a population-based community sample of older adults with Type 2 diabetes. J Behav Med. 2012;35:190–199. doi: 10.1007/s10865-011-9344-6. [DOI] [PubMed] [Google Scholar]
- 17.West R.K., Ravona-Springer R., Schmeidler J., Leroith D., Koifman K., Guerrero-Berroa E. The association of duration of type 2 diabetes with cognitive performance is modulated by long-term glycemic control. Am J Geriatr Psychiatry. 2014;22:1055–1059. doi: 10.1016/j.jagp.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kalmijn S., Feskens E.J., Launer L.J., Stijnen T., Kromhout D. Glucose intolerance, hyperinsulinaemia and cognitive function in a general population of elderly men. Diabetologia. 1995;38:1096–1102. doi: 10.1007/BF00402181. [DOI] [PubMed] [Google Scholar]
- 19.Hiltunen L., Keinänen-Kiukaanniemi S., Läärä E. Glucose tolerance and cognitive impairment in an elderly population. Public Health. 2001;115:197–200. doi: 10.1038/sj/ph/1900758. [DOI] [PubMed] [Google Scholar]
- 20.U'Ren R.C., Riddle M.C., Lezak M.D., Bennington-Davis M. The mental efficiency of the elderly person with type II diabetes mellitus. J Am Geriatr Soc. 1990;38:505–510. doi: 10.1111/j.1532-5415.1990.tb02398.x. [DOI] [PubMed] [Google Scholar]
- 21.Action to Control Cardiovascular Risk in Diabetes Study Group Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545–2559. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murray A.M., Hsu F.C., Williamson J.D., Bryan R.N., Gerstein H.C., Sullivan M.D. ACCORDION MIND: results of the observational extension of the ACCORD MIND randomised trial. Diabetologia. 2017;60:69–80. doi: 10.1007/s00125-016-4118-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bahar-Fuchs A., Clare L., Woods B. Cognitive training and cognitive rehabilitation for mild to moderate Alzheimer's disease and vascular dementia. Cochrane Database Syst Rev. 2013;6:CD003260. doi: 10.1002/14651858.CD003260.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Belleville S. Cognitive training for persons with mild cognitive impairment. Int Psychogeriatr. 2008;20:57–66. doi: 10.1017/S104161020700631X. [DOI] [PubMed] [Google Scholar]
- 25.Gates N.J., Sachdev P.S., Fiatarone Singh M.A., Valenzuela M. Cognitive and memory training in adults at risk of dementia: a systematic review. BMC Geriatr. 2011;11:55. doi: 10.1186/1471-2318-11-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hill N.T., Mowszowski L., Naismith S.L., Chadwick V.L., Valenzuela M., Lampit A. Computerized cognitive training in older adults with mild cognitive impairment or dementia: a systematic review and meta-analysis. Am J Psychiatry. 2017;174:329–340. doi: 10.1176/appi.ajp.2016.16030360. [DOI] [PubMed] [Google Scholar]
- 27.Kelly M.E., Loughrey D., Lawlor B.A., Robertson I.H., Walsh C., Brennan S. The impact of cognitive training and mental stimulation on cognitive and everyday functioning of healthy older adults: a systematic review and meta-analysis. Ageing Res Rev. 2014;15:28–43. doi: 10.1016/j.arr.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 28.Kueider A.M., Parisi J.M., Gross A.L., Rebok G.W. Computerized cognitive training with older adults: a systematic review. PLoS One. 2012;7:e40588. doi: 10.1371/journal.pone.0040588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lampit A., Hallock H., Valenzuela M. Computerized cognitive training in cognitively healthy older adults: a systematic review and meta-analysis of effect modifiers. PLoS Med. 2014;11:e1001756. doi: 10.1371/journal.pmed.1001756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chapman S.B., Aslan S., Spence J.S., Hart J.J. Jr, Bartz E.K., Didehbani N. Neural mechanisms of brain plasticity with complex cognitive training in healthy seniors. Cereb Cortex. 2013;25:396–405. doi: 10.1093/cercor/bht234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Valenzuela M.J., Breakspear M., Sachdev P. Complex mental activity and the aging brain: molecular, cellular and cortical network mechanisms. Brain Res Rev. 2007;56:198–213. doi: 10.1016/j.brainresrev.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 32.Bahar-Fuchs A., Kudlicka A., Clare L. Cognitive rehabilitation for people with dementia: What is it and does it work? Aust J Dement Care. 2016;5:37–40. [Google Scholar]
- 33.Bahar-Fuchs A., Clare L., Woods B. Cognitive training and cognitive rehabilitation for mild to moderate Alzheimer's disease and vascular dementia. Cochrane Libr. 2013;6:1–103. doi: 10.1002/14651858.CD003260.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bandura A. Self-efficacy: toward a unifying theory of behavioral change. Psychol Rev. 1977;84:191. doi: 10.1037//0033-295x.84.2.191. [DOI] [PubMed] [Google Scholar]
- 35.Boot W.R., Champion M., Blakely D.P., Wright T., Souders D.J., Charness N. Video games as a means to reduce age-related cognitive decline: attitudes, compliance, and effectiveness. Front Psychol. 2013;4:31. doi: 10.3389/fpsyg.2013.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nyenhuis N., Zastrutzki S., Jäger B., Kröner-Herwig B. An internet-based cognitive-behavioural training for acute tinnitus: secondary analysis of acceptance in terms of satisfaction, trial attrition and non-usage attrition. Cogn Behav Ther. 2013;42:139–145. doi: 10.1080/16506073.2012.724081. [DOI] [PubMed] [Google Scholar]
- 37.Cane J., O'Connor D., Michie S. Validation of the theoretical domains framework for use in behaviour change and implementation research. Implement Sci. 2012;7:37. doi: 10.1186/1748-5908-7-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Michie S., van Stralen M.M., West R. The behaviour change wheel: a new method for characterising and designing behaviour change interventions. Implementation Sci. 2011;6:42. doi: 10.1186/1748-5908-6-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Michie S., Richardson M., Johnston M., Abraham C., Francis J., Hardeman W. The behavior change technique taxonomy (v1) of 93 hierarchically clustered techniques: building an international consensus for the reporting of behavior change interventions. Ann Behav Med. 2013;46:81–95. doi: 10.1007/s12160-013-9486-6. [DOI] [PubMed] [Google Scholar]
- 40.Sheeran P., Maki A., Montanaro E., Avishai-Yitshak A., Bryan A., Klein W.M. The Impact of Changing Attitudes, Norms, and Self-efficacy on Health-Related Intentions and Behavior: A Meta-analysis. Health Psych. 2016;11:1178–1188. doi: 10.1037/hea0000387. [DOI] [PubMed] [Google Scholar]
- 41.Beeri M.S., Ravona-Springer R., Moshier E., Schmeidler J., Godbold J., Karpati T. The Israel Diabetes and Cognitive Decline (IDCD) study: design and baseline characteristics. Alzheimers Dement. 2014;10:769–778. doi: 10.1016/j.jalz.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bahar-Fuchs A., Webb S., Bartsch L., Clare L., Rebok G., Cherbuin N. Tailored and adaptive computerized cognitive training in older adults at risk for dementia: A randomized controlled trial. J Alzheimers Dis. 2017;60:889–911. doi: 10.3233/JAD-170404. [DOI] [PubMed] [Google Scholar]
- 43.Gigler K.L., Blomeke K., Shatil E., Weintraub S., Reber P.J. Preliminary evidence for the feasibility of at-home online cognitive training with older adults. Gerontechnology. 2013;12:26–35. doi: 10.4017/gt.2013.12.1.007.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Haimov I., Hanuka E., Horowitz Y. Chronic insomnia and cognitive functioning among older adults. Behav Sleep Med. 2008;6:32–54. doi: 10.1080/15402000701796080. [DOI] [PubMed] [Google Scholar]
- 45.Horowitz-Kraus T., Vannest J.J., Kadis D., Cicchino N., Wang Y.Y., Holland S.K. Reading acceleration training changes brain circuitry in children with reading difficulties. Brain Behav. 2014;4:886–902. doi: 10.1002/brb3.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Peretz C., Korczyn A.D., Shatil E., Aharonson V., Birnboim S., Giladi N. Computer-based, personalized cognitive training versus classical computer games: a randomized double-blind prospective trial of cognitive stimulation. Neuroepidemiology. 2011;36:91–99. doi: 10.1159/000323950. [DOI] [PubMed] [Google Scholar]
- 47.Preiss M., Shatil E., Čermáková R., Cimermanová D., Ram I. Personalized cognitive training in unipolar and bipolar disorder: a study of cognitive functioning. Front Hum Neurosci. 2013;7:108. doi: 10.3389/fnhum.2013.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ansteya K.J., Bahar-Fuchs A., Heratha P., Kima S., Burnsa R., Rebok G.W. Body brain life: A randomized controlled trial of an online dementia risk reduction intervention in middle-aged adults at risk of Alzheimer's disease. Alzheimers Dement (N Y) 2015;1:72–80. doi: 10.1016/j.trci.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gaitán A., Garolera M., Cerulla N., Chico G., Rodriguez-Querol M., Canela-Soler J. Efficacy of an adjunctive computer-based cognitive training program in amnestic mild cognitive impairment and Alzheimer's disease: a single-blind, randomized clinical trial. Int J Geriatr Psychiatry. 2012;28:91–99. doi: 10.1002/gps.3794. [DOI] [PubMed] [Google Scholar]
- 50.Maddux J.E., Lewis J. Springer; 1995. Self-efficacy and Adjustment, in Self-efficacy, Adaptation, and Adjustment; pp. 37–68. [Google Scholar]
- 51.Rebok G.W., Ball K., Guey L.T., Jones R.N., Kim H.Y., King J.W. Ten-year effects of the ACTIVE cognitive training trial on cognition and everyday functioning in older adults. J Am Geriatr Soc. 2014;62:16–24. doi: 10.1111/jgs.12607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schmitt A., Gahr A., Hermanns N., Kulzer B., Huber J., Haak T. The Diabetes Self-Management Questionnaire (DSMQ): development and evaluation of an instrument to assess diabetes self-care activities associated with glycaemic control. Health Qual Life Outcomes. 2013;11:138. doi: 10.1186/1477-7525-11-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zeidner M., Schwarzer R., Jerusalem M. Hebrew adaptation of the general self-efficacy scale. Health Psychol. 1993;12:102–104. [Google Scholar]
- 54.Mishali M., Omer H., Heymann A. The importance of measuring self-efficacy in patients with diabetes. Fam Pract. 2010;28:82–87. doi: 10.1093/fampra/cmq086. [DOI] [PubMed] [Google Scholar]
- 55.Troyer A.K., Rich J.B. Psychometric properties of a new metamemory questionnaire for older adults. J Gerontol B Psychol Sci Soc Sci. 2002;57:P19–P27. doi: 10.1093/geronb/57.1.p19. [DOI] [PubMed] [Google Scholar]
- 56.Yesavage J.A., Sheikh J.I. 9/Geriatric depression scale (GDS) recent evidence and development of a shorter version. Clin Gerontol. 1986;5:165–173. [Google Scholar]
- 57.Pachana N.A., Byrne G.J., Siddle H., Koloski N., Harley E., Arnold E. Development and validation of the Geriatric Anxiety Inventory. Int Psychogeriatr. 2007;19:103–114. doi: 10.1017/S1041610206003504. [DOI] [PubMed] [Google Scholar]
- 58.Bucks R.S., Ashworth D.L., Wilcock G.K., Siegfried K. Assessment of activities of daily living in dementia: development of the Bristol Activities of Daily Living Scale. Age Ageing. 1996;25:113–120. doi: 10.1093/ageing/25.2.113. [DOI] [PubMed] [Google Scholar]
- 59.Morris J.C. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 60.Zarit S.H., Reever K.E., Bach-Peterson J. Relatives of the impaired elderly: correlates of feelings of burden. Gerontologist. 1980;20:649–655. doi: 10.1093/geront/20.6.649. [DOI] [PubMed] [Google Scholar]
- 61.Kaufer D.I., Cummings J.L., Ketchel P., Smith V., MacMillan A., Shelley T. Validation of the NPI-Q, a brief clinical form of the Neuropsychiatric Inventory. J Neuropsychiatry Clin Neurosci. 2000;12:233–239. doi: 10.1176/jnp.12.2.233. [DOI] [PubMed] [Google Scholar]
- 62.Cummings J.L., Mega M., Gray K., Rosenberg-Thompson S., Carusi D.A., Gornbein J. The Neuropsychiatric Inventory comprehensive assessment of psychopathology in dementia. Neurology. 1994;44:2308. doi: 10.1212/wnl.44.12.2308. [DOI] [PubMed] [Google Scholar]
- 63.Carpenter J.R., Kenward M.G. John Wiley & Sons; 2013. Missing Data in Clinical Trials–A Practical Guide. 2008; p. 364. [Google Scholar]
- 64.Seltman H.J. Experimental Design and Analysis. 2015. Mixed models. A flexible approach to correlated data; pp. 357–377. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.