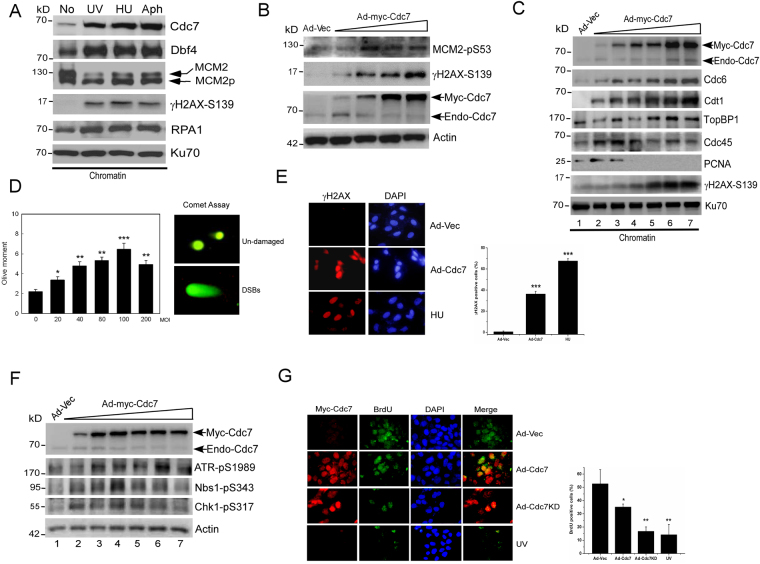
Figure 1.
Cdc7 overexpression causes DNA lesions and activates ATM/ATR-mediated checkpoint. (A) Cdc7 and Dbf4 accumulate on stalled fork to activate more MCM2 phosphorylation and cause the formation of DNA lesions upon replication stress. Chromatin fractions were isolated from U2OS cells after mock treatment (No), or UV (50 J/m2), HU (1 mM, 24 h after), or Aphidicolin (Aph, 1 μg/ml, 24 h after) and were applied to immunoblotting. Equal amounts of input protein were subjected to immunoblotting using anti-Cdc7, anti-Dbf4, anti-MCM2, anti-γ-H2AX, and anti-RPA1 antibodies. Ku70 was served as a loading control. (B) Cdc7 overexpression induces DSB formation. U2OS was infected with Ad-Cdc7 (0.6, 1.2, 2.4, or 3.6 × 108 pfu/ml) or Ad-Vec (3.6 × 108 pfu/ml) for 48 h. The lysates were analyzed by Western blotting using the indicated antibodies. The expression of endogenous Cdc7 (Endo-Cdc7) and adenovirally introduced Myc tagged Cdc7 (Myc-Cdc7) is shown as indicated. (C) Cdc7 overexpression triggers the initiation of DNA replication and causes DSB formation that activates S-checkpoint. U2OS was infected with Ad-Cdc7 (0.3, 0.6, 1.2, 2.4, 3.6, or 7.2 × 108 pfu/ml) or Ad-Vec (3.6 × 108 pfu/ml) for 48 h. Chromatin fractions were isolated and analyzed by immunoblotting. Equal amounts of input protein were subjected to immunoblotting using anti-Cdc7, anti-Cdc6, anti-Cdt1, anti-TopBP1, anti-Cdc45, anti-PCNA, and anti-γ-H2AX antibodies. Ku70 was served as a loading control. The expression of endogenous Cdc7 (Endo-Cdc7) and adenovirally introduced Myc tagged Cdc7 (Myc-Cdc7) is shown. (D) Comet assays indicate DSB formation when Cdc7 is overexpressed. U2OS was infected with Ad-Cdc7 using different viral titers (0.6 × 108 pfu/ml, MOI = 20; 1.2 × 108 pfu/ml, MOI = 40; 2.4 × 108 pfu/ml, MOI = 80; 3.6 × 108 pfu/ml, MOI = 100; 7.2 × 108 pfu/ml, MOI = 200) or Ad-GFP control. Olive moment for each condition was measured using the Comet Score software (TriTek Corporation, Sumerduck, VA, USA). Fifty cells were counted for each experiment. Two typical cells with or without comet tails are shown on the right. Results are presented as range of three independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001. (E) Cdc7 overexpression triggers γH2AX formation by immunofluorescence analysis. U2OS cells were infected with Ad-Cdc7 (2.4 × 108 pfu/ml) or Ad-Vec for 48 h and immunostaining was performed by using antibodies toward γH2AX. U2OS cells treated with hydroxyurea (HU, 1 mM) for 16 h only were used as a positive control. DAPI was used for nuclear staining. The graph with error bars is shown in the right panel. Results are presented as range of three independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001. (F) Cdc7 overexpression activates ATR-mediated checkpoint. U2OS was infected with Ad-Cdc7 (0.3, 0.6, 1.2, 2.4, 3.6, or 7.2 × 108 pfu/ml) or Ad-Vec (3.6 × 108 pfu/ml) for 48 h. The lysates were analyzed by immunoblotting using anti-Cdc7, anti-phospho-ATR (S1989), anti-phospho-Nbs1 (S343), and anti-phospho-Chk1 (S317) antibodies. β-actin was served as a loading control. The expression of endogenous Cdc7 (Endo-Cdc7) and adenovirally introduced Myc tagged Cdc7 (Myc-Cdc7) is shown. (G) Cdc7 overexpression reduces BrdU incorporation. U2OS cells were infected with Ad-Cdc7 (2.4 × 108 pfu/ml), Ad-Cdc7KD (2.4 × 108 pfu/ml), or Ad-Vec (2.4 × 108 pfu/ml) for 48 h, and immunostaining was performed by using antibodies toward BrdU and Myc. U2OS cells treated with UV irradiation (50 J/m2) only were used as a positive control. DAPI was used for nuclear staining. The graph with error bars indicating the standard error from the average is shown in the right panel. Results are presented as range of three independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001.