Abstract
Due to antibacterial characteristic, amnion has been frequently used in different clinical situations. Developing an in vitro method to augment endogenous antibacterial ingredient of amniotic epithelial and mesenchymal stem cells is desirable for a higher efficacy of this promising biomaterial. In this study, epithelial or mesenchymal side dependent effect of amniotic membrane (AM) on antibacterial activity against some laboratory and clinical isolated strains was investigated by modified disk diffusion method and colony count assay. The effect of exposure to IL-1β in production and release of antibacterial ingredients was investigated by ELISA assay. The results showed that there is no significant difference between epithelial and mesenchymal sides of amnion in inhibition of bacterial growth. Although the results of disk diffusion showed that the AM inhibitory effect depends on bacterial genus and strain, colony count assay showed that the extract of AM inhibits all investigated bacterial strains. The exposure of AM to IL-1β leads to a higher level of antibacterial peptides secretion including elafin, HBD-2, HBD-3 and cathelicidic LL-37. Based on these results, amniotic cells possess antibacterial activity which can be augmented by inflammatory signal inducers; a process which make amnion and its epithelial and mesenchymal stem cells more suitable for tissue engineering and regenerative medicine.
Introduction
Extra-embryonic membrane is one of the most frequently used sources for regenerative medicine. Amnion is the innermost part of this membrane which has five layers1. The amnion consists two distinct cell population; Epithelial cells and Mesenchymal cells with characteristics of stem cells2. The amniotic epithelial cells contain non-intestinal-type surface microvilli, intracytoplasmic lumina lined with microvilli, and intercellular junctions; and amniotic mesenchymal cells have rough endoplasmic reticulum profiles, lipid droplets, and well-developed foci of contractile filaments with dense bodies3. Furthermore, the extracellular matrix of amnion (which mostly secreted by amniotic epithelial and mesenchymal cells) in the basement membrane and compact layer contains collagen, laminin1, elastin, fibronectin, vitronectin, nidogen4, and hyaluronic acid5, which are important for using the AM as a natural scaffold in tissue engineering and regenerative medicine1,6,7.
The amniotic membrane (AM) possesses different characteristics including low immunogenicity8, anti-inflammatory effect9, angiogenic modulatory properties10,11, anti-cancer properties12 and antimicrobial activity13; which make it suitable for clinical situations such as ophthalmology14.
Amnion antimicrobial activity is caused by peptides such as defensins, SLPI (Secretory Leukocyte Protease Inhibitor), and elafin, which are often displayed in mucosal surface and synthesized by amniotic epithelial and mesenchymal cells15. Natural antibacterial peptides are necessary for innate immune system which protect pregnancy against bacterial, fungal and viral infections16,17. Maternal infection during the pregnancy and within delivery can cause preterm delivery and disorders or diseases in fetal and newborn18. To prevent the effect of contaminations during pregnancy and delivery, uterus, endometrium19, placenta and fetal membranes secrete natural antimicrobial peptides20.
Human amnion is one of the tissues which produces and secretes antimicrobial peptides. Human defensins are the first group of these peptides which are secreted in mucosal tissues21. Human beta-defensin 2 and 3 display regularly in the amniotic cells22 by being exposed to the Gram negative and Gram positive bacteria23. Elafin is the other amnion antimicrobial peptide that act as anti-protease20,24 and elastase inhibitor25,26. Additionally, the AM possesses some natural elements like hyaluronic acid5, lactoferrin, Cathelicidin, and interleukin-1 receptor antagonist that decrease inflammation and bacterial infection27,28.
The main aim of this article was to study the epithelial and mesenchymal side dependent antibacterial effect of fresh amnion on different bacterial strains. Moreover, this study focused on secretory state of amniotic cell ingredients which can be induced by inflammatory stimuli such as IL-1β or bacterial LPS; a procedure which boosts antimicrobial capability of amniotic membrane in regenerative medicine.
Materials and Methods
Amnion preparation
All Methods and experimental procedures of this study were in accordance with the experimental guidelines and regulations and approved by the research ethic committee of Shahid Beheshti University of Medical Sciences. Human placenta (n = 30) was collected immediately after the elective Caesarean delivery under sterile conditions and treated with cold normal saline to be transferred to laboratory. Placentas were obtained from healthy women at gestational ages 36–38 weeks with serologically negative results for HIV, HBV, HCV and syphilis. Informed consent was obtained from the parents.
Amnion was separated from the chorion by peeling in sterile condition under laminar flow. Then amnion was washed with cold phosphate buffered saline (PBS) three times to remove blood residue.
Microbial assay
Antibacterial property of amnion was measured by modified disk diffusion method. This method was adapted from the Clinical and Laboratory Standard Institute (CLSI) protocol. Pseudomonas aeruginosa ATCC 27853, Staphylococcus aureus ATCC 25923, Escherichia coli ATCC 25922 and two clinically isolated sensitive strains of E. coli (T3 and T4) were used for antibacterial susceptibility test.
All bacterial strains were cultured on the blood agar medium (Merck, Germany). After being incubated overnight at 35 ± 2 °C, some colonies were harvested from plates and separately suspended in the sterile normal saline. The turbidity was equivalent to a 0.5 McFarland standard. Finally, these bacteria were cultured on the Muller-Hinton agar plates (Merck, Germany).
After being isolated and washed, the fresh amnion was cut into small pieces (1 × 1 cm). Each of the pieces were placed on the seeded Muller-Hinton agar plate in 20–30 minutes after separation, according to the modified disk diffusion method13,29. Amnion pieces were put separately in two ways, epithelial side up and mesenchymal side up as an antibacterial peptide releasing disk. Then the plates were incubated at 35 ± 2 °C overnight and the inhibitory effects were measured.
In order to avoid probable contamination within processing and preparation of the AM, a small piece (1 × 1 cm) of fresh AM was put on the Muller-Hinton agar plates and microbial growth was controlled 24 h after cultivation of tissues.
Amnion extract antibacterial effect
Antibacterial effect of amnion extract was examined with a modified method in which a piece of amnion (1 × 1 cm) was cut into smaller sections and added to equivalent volume of PBS. The extract was obtained by sonicating the amnion on ice for 10 minutes with 80 W and 0.5 s cycle (Hielscher, Ultrasound Technology, Germany). Then membrane residues were removed by centrifuging at 800 rpm for 4 min. 500 μl of amnion extract was added to the surface of each Muller-Hinton agar plates. After drying the surface of plates, the bacterial inoculum was added to them. The number of colonies was counted after overnight incubation at 35 ± 2 °C.
Evaluating the secretory state of natural antimicrobial peptides
To evaluate secretory state of antimicrobial agents from amniotic cells, the amniotic membrane was cut into 12 small pieces that were each 2 × 2 cm, and the pieces were individually cultured in a 12-well plate. To each well, 1 mL of DMEM/F12 containing FBS 10% and penicillin-streptomycin 1% was added. The plates were incubated in 37 °C with 5% CO2.
After 24 hours, medium was changed to serum-depleted (1% FBS) for 20 hours before treatments were added; 1% FBS was used because our previous study demonstrated decreased amniotic cell viability after 24 hours of culture in the absence of serum30. Half of the plates were treated with 10 ng/mL recombinant human interleukin (IL)−1beta (Peprotech, London, UK) to induce production and secretion of natural antimicrobial. The plates were incubated in 37 °C with 5% CO2 for 24 h, after which the supernatant was collected from each well.
Enzyme-linked immunosorbent assay
Elafin, HBD-2, HBD-3 and cathelicidin LL-37 concentrations in the supernatant of amniotic membrane cultures were determined by elafin ELISA kit (Abcam, USA), HBD-2 assay kit (PeproTech) and HBD-3 and Cathelicidin LL-37 ELISA kits (HyCult Biotech), according to company instructions.
Statistical analysis
Statistical comparisons were performed as mean ± standard error of the mean (SEM). Statistical significance was determined by means of one-way analysis of variance (ANOVA) followed by Tukey’s post-test. A P-value less than 0.05 was considered statistically significant.
Data availability
All data generated or analyzed during this study are included in this published article.
Results
Possible bacterial contaminations of membranes were controlled by culturing the fresh tissues on Muller-Hinton agar plates and microbiologically proper samples without bacterial contaminations were used for data interpretation. Totally, 30 amnion were examined in this study and at least 6 amnion were used for each bacterial strain in epithelial or mesenchymal sides separately. In the statistical analysis, only the groups with 50% inhibition zone in cultured plates were used.
Based on the disk diffusion method, the inhibitory effect of the AM was appeared in P. aeruginosa ATCC 27853, E. coli T3 and T4 after 24 hours of incubation (Table 1).
Table 1.
The inhibitory effects of epithelial side up and mesenchymal side up amnion on three bacterial strains based on the disk diffusion method.
| Bacterial strain | Amnion | |||||||
|---|---|---|---|---|---|---|---|---|
| Epithelial side up | Mesenchymal side up | |||||||
| P. aeruginosa ATCC 27853 | Inhibition zone (n = 14)a | Elimination under tissue (n = 14)b | Mean inhibition zone (n = 14) | Max. inhibition zone | Inhibition zone (n = 14)a | Elimination under tissue (n = 14)b | Mean inhibition zone (n = 14) | Max. inhibition zone |
| 12c, 2d | 14 | 2.8 mm | 5 mm | 14 | 13c, 1d | 2 mm | 4 mm | |
| E. coli T3 | Inhibition zone (n = 21)a | Elimination under tissue (n = 19)b | Mean inhibition zone (n = 21) | Max. inhibition zone | Inhibition zone (n = 17)a | Elimination under tissue (n = 17)b | Mean inhibition zone (n = 21) | Max. inhibition zone |
| 19c, 2d | 14c, 5d | 1.2 mm | 2 mm | 16c, 1d | 14c, 3d | 1 mm | 1 mm | |
| E. coli T4 | Inhibition zone (n = 14) | Elimination under tissue (n = 15) | Mean inhibition zone (n = 14) | Max. inhibition zone | Inhibition zone (n = 22)a | Elimination under tissue (n = 14)b | Mean inhibition zone (n = 14) | Max. inhibition zone |
| 12c, 2d | 12c, 3d | 1.3 mm | 2 mm | 19c, 3d | 12c, 2d | 1 mm | 2 mm | |
aThe number of plates examined for inhibition zone.
bThe number of plates examined for elimination of bacterial growth under tissue.
cThe number of plate with inhibition zone or elimination.
dThe number of plate without inhibition zone or elimination.
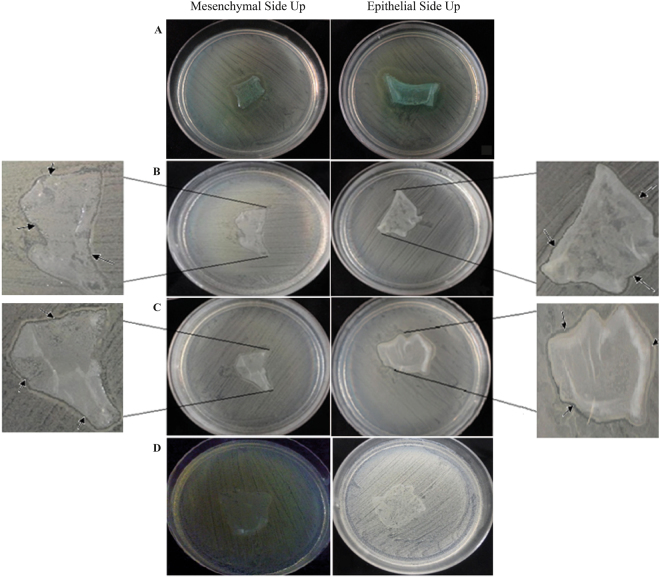
The growth was decreased under and in the edge of the tissues in both epithelial side and mesenchymal side up amnion. The mean of inhibition zone was about 2 mm in the epithelial side up AM and 2.8 mm in mesenchymal side up AM for P. aeruginosa ATCC 27853 cultures. The maximum inhibition (5 mm) was seen in this group (Fig. 1a) (Table 1).
Figure 1.
The inhibition zone of mesenchymal side up and epithelial side up amnion in, (a) P. aeruginosa ATCC 27853 culture, (b) E. coli T3 culture (The arrows indicate the inhibition zone), (c) E. coli T4 culture (The arrows indicate the inhibition zone), (d) No inhibitory effect appeared in; E. coli ATCC 25922 (left) and S. aureus ATCC 25923 (right). The experiment was performed in triplicate (n = 5 for each time).
The inhibitory effect among the E. coli strains demonstrated different results. The inhibition zone was appeared in two sensitive clinical isolated E. coli (T3 and T4) in both epithelial and mesenchymal side up. Figure 1b and Fig. 1c demonstrate the inhibition zone in E. coli T3 and E. coli T4, respectively.
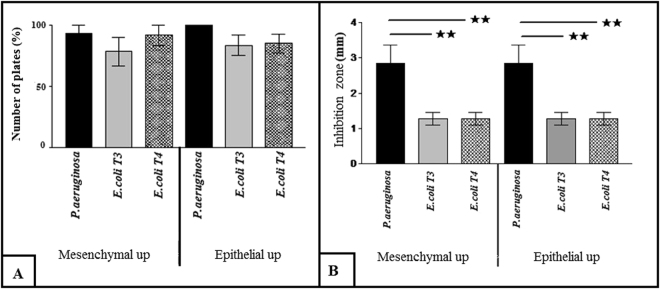
Statistical analysis showed that there is no significant difference in the number of plates in which the inhibitory effect was seen against P. aeruginosa ATCC 27853, E. coli T3 and T4 (Fig. 2). However, the inhibition zone in P. aeruginosa ATCC 27853 is significantly (P < 0.01) greater than two clinical isolated E. coli T3 and T4 (Fig. 2).
Figure 2.
(a) The number of plates (%) with inhibition zone in bacterial culture in epithelial side up and mesenchymal side up amnion; (b) The mean of inhibition zone (mm) in epithelial side up and mesenchymal side up amnion in bacterial culture after overnight incubation (P < 0.01★★). The experiment was performed in quadruplicate (n = 5 for each time).
To investigate the effects of amnion side on antibacterial activity, we compared the results between mesenchymal and epithelial sides of amnion. As shown in Fig. 2, there is no significant difference between the number of plates and inhibition zone of P. aeruginosa ATCC 27853 which incubated with mesenchymal side amnion in comparison to those of incubated with epithelial side amnion. The same results were achieved from incubation of E. coli T3 and T4 with both mesenchymal and epithelial sides of amnion.
No inhibitory effect was seen in E. coli ATCC 25922 and S. aureus ATCC 25923 after 24 hours of incubation under and in the edge of both epithelial side up and mesenchymal side up amnion (Fig. 1d).
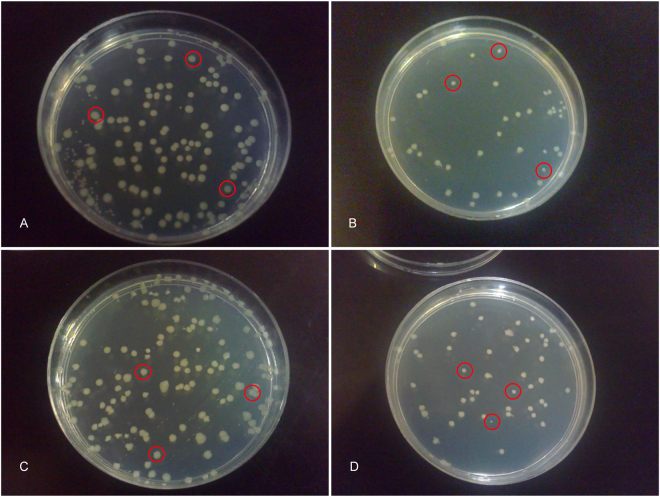
In order to consider the effects of the contents of amniotic cells, the AMs were sonicated and the inhibitory effects of amnion extract were evaluated on P. aeroginosa ATCC 27853, S. aureus ATCC 25923, E. coli ATCC 25922, and two clinical sensitive strains of E. coli (T3, T4). Consistent with the results of disk diffusion method, the number of colonies decreased in P. aeroginosa ATCC 27853 and two clinical sensitive strains of E. coli (T3, T4) significantly in comparison to control groups (Fig. 3). Surprisingly, the extract of amnion significantly reduced the number of colonies of S. aureus ATCC 25923 and E. coli ATCC25922. In addition to the number of colonies, the extract of amnion decreased the size of S. aureus ATCC 25923 and E. coli ATCC25922 colonies compared to the control groups (Fig. 4).
Figure 3.
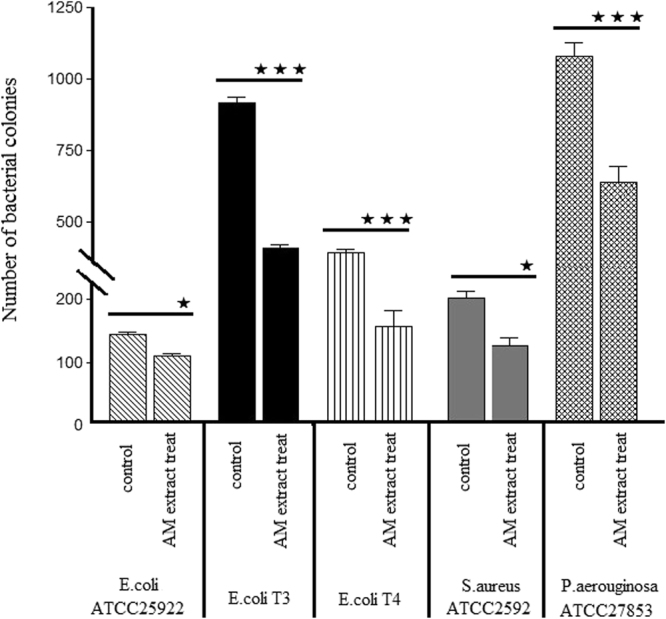
The number of bacterial colonies appeared in Muller-Hinton agar plates treated with amnion extract (P < 0.05 ★, P < 0.001 ★★★). The experiment was performed in quadruplicate (n = 5 for each time).
Figure 4.
The extract of amnion decreased the size and number of (a) S. aureus ATCC 25923 and (c) E. coli ATCC25922 colonies compared to the control groups (b and d, respectively). The reduced size of colonies is shown by red circles. The experiment was performed in triplicate (n = 5 for each time).
To evaluate secretory state of amniotic cells after induction with IL-1β, elafin, HBD-2, HBD-3 and cathelicidin LL-37 protein levels were measured in amniotic membrane culture supernatant by ELISA. The rational for choosing IL-1 beta as pro-inflammatory cytokine was a pilot study in which we compared 10 ng/ml of TNF-alpha with the same concentration of IL-1 beta in production of HBD-3 at time points 6, 12, 24 and 48 hours. Although the primary results showed that both pro-inflammatory cytokines significantly up-regulated HBD-3 production compared to unstimulated control, HBD-3 production was significantly higher at each time point in response to IL-1 beta (data not shown). As evaluated by trypan blue exclusion, treatments with IL-1β had no significant effect on amniotic cell numbers or viability after 24 hours.
As shown in Fig. 5, amniotic membrane releases a basal level of antimicrobial peptides elafin, HBD-2, HBD-3 and cathelicidin LL-37 in the culture medium. Significantly higher levels of elafin, HBD-2, HBD-3 and cathelicidin LL-37 proteins were secreted by the membranes treated with IL-1β 10 ng/mL for 24 hours.
Figure 5.
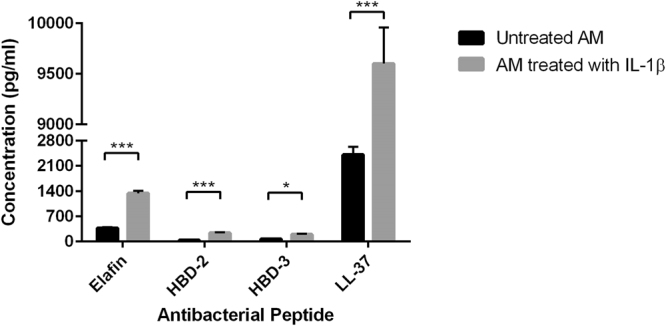
Antibacterial peptides concentration in the AM culture supernatant before and after treatment with IL-1β for 24 hours (P < 0.05 ★, P < 0.001 ★★★). The experiment was performed in quadruplicate (n = 5 for each time).
Discussion
In this study, the antibacterial property of amnion was examined on different bacterial strains and the side dependent effect of amniotic membrane was also investigated on antibacterial activity. Furthermore, the amnion extract was used to optimize the inhibitory effect.
Disk diffusion is a commonly used method to evaluate antibacterial property of the AM. Talmi et al. used the modified disk diffusion method for investigating the amniotic membrane antimicrobial activity on coagulase positive staphylococcus, E. coli, Kelebsiella pneumonias, P. aeruginosa, Proteus mirabilis. They demonstrated that the inhibition zone appears exactly under the tissue due to the close contact between amnion and culture plate13. In further studies, Kjaegaarda et al. flattened the fresh amnion on the nitrocellulose filter and utilized it in vitro study. They reported that a narrow inhibition zone (1 mm) appeared in Group A streptococcus, Staphylococcus saprophyticus, E. coli, P. aeruginosa and S. aureus 31. We also previously demonstrated the antibacterial properties of cryopreserved AM by this method32.
In the present study, we used both standard and clinical bacterial strains to investigate antibacterial property by disk diffusion method. In accordance to the results of disk diffusion method, the inhibitory effect of fresh amnion appeared in P. aeruginosa and two clinical isolated strains of E. coli (T3 and T4) opposed to S. aureus ATCC 25923 and E. coli ATCC 25922. The results of disk diffusion method showed that the amnion inhibitory effect was dependent on bacterial genus and bacterial strain. According to our results, it seems that the inhibitory effect in disk diffusion method is due to either the contact between the amnion and bacterial strains13 or achieving a secretion threshold of antibacterial ingredients which is enough to inhibit the bacterial activity of some strains. For instance, elafin can inhibit serine peptidase that is a virulence factor in P. aeruginosa in a critical threshold concentration which is not enough to inhibit growth of the other bacterial strains33,34. On the other hand, using the amnion extract reduced the size and number of colonies in all strains. In the case of two strains which were not inhibited in disk diffusion method but inhibited using the amnion extract (S. aureus ATCC 25923 and E. coli ATCC 25922), we have two options. Either amniotic membrane cannot release its ingredients in disk diffusion condition or the amniotic antibacterial ingredients are non-secretory. As shown in results, exposing the AM to IL-1β resulted in a higher amount of elafin, HBD-2, and HBD-3 and cathelicidin LL-37 secretion which confirmed secretory state of amniotic antibacterial ingredients. This result is consistent with previous study in which we showed that AM releases some anti-cancer and immune-modulatory agents in condition medium35,36. Therefore, the non-secretory state of amniotic antibacterial ingredients is not acceptable and it seems that the disk diffusion method does not provide an appropriate condition for amnion to display its antibacterial property. To the best of our knowledge, disk diffusion method is the most common method for assessing the AM antibacterial property; and since it is not a proper method for this aim, it is necessary to modify and develop alternative methods to attain a reliable method for investigating the antibacterial property of AM.
In this study the side dependent effect of amnion on bacterial activity was examined. There are no significant difference between epithelial side up and mesenchymal side up amnion in inhibitory effect. This similarity in two sides of amnion suggests that not only amniotic epithelial cells cause inhibitory effect, but also amnion mesenchymal cells can display antibacterial property. Although all previous studies focused on antibacterial properties of amniotic epithelial cells, amniotic mesenchymal cells also could have antibacterial characteristics32,37. Mesenchymal stem cells have antibacterial characteristics. It has been shown that lipocalin 238, beta-defensin 239 and LL-37 (human cathelicidin antimicrobial peptide, hCAP-18)40 in mesenchymal stem cells from the other sources such as bone marrow and adipose demonstrate antibacterial property against bacterial strains; hence, the amniotic mesenchymal cells also may contribute to inhibitory effect of amniotic membrane. More studies would be required to determine the antibacterial effect of amniotic mesenchymal cells.
Acknowledgements
The authors would like to thank Prof. H. Peirovi and the operation room personnel of Erfan hospital for their assistance in this research. This work was financially supported by deputy for research affairs of Shahid Beheshti University of Medical Sciences (stem cells network grant).
Author Contributions
H.N. and F.T. designed the study. H.N., F.T., and K.M. performed the experiments. H.N., K.M., and S.A. wrote the manuscript. H.N. and K.M. revised the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Fatemeh A. Tehrani, Khashayar Modaresifar and Sara Azizian contributed equally to this work.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Niknejad H, et al. Properties of the amniotic membrane for potential use in tissue engineering. Eur Cells Mater. 2008;15:88–99. doi: 10.22203/eCM.v015a07. [DOI] [PubMed] [Google Scholar]
- 2.Ilancheran S, et al. Stem cells derived from human fetal membranes display multilineage differentiation potential. Biol. Reprod. 2007;77:577–588. doi: 10.1095/biolreprod.106.055244. [DOI] [PubMed] [Google Scholar]
- 3.Hu J, Cai Z, Zhou Z. Progress in studies on the characteristics of human amnion mesenchymal cells. Prog Nat Sci. 2009;19:1047–1052. doi: 10.1016/j.pnsc.2008.12.005. [DOI] [Google Scholar]
- 4.Riau AK, Beuerman RW, Lim LS, Mehta JS. Preservation, sterilization and de-epithelialization of human amniotic membrane for use in ocular surface reconstruction. Biomaterials. 2010;31:216–225. doi: 10.1016/j.biomaterials.2009.09.034. [DOI] [PubMed] [Google Scholar]
- 5.Higa K, Shimmura S, Shimazaki J, Tsubota K. Hyaluronic acid-CD44 interaction mediates the adhesion of lymphocytes by amniotic membrane stroma. Cornea. 2005;24:206–212. doi: 10.1097/01.ico.0000133999.45262.83. [DOI] [PubMed] [Google Scholar]
- 6.Kakavand M, Yazdanpanah G, Ahmadiani A, Niknejad H. Blood compatibility of human amniotic membrane compared with heparin‐coated ePTFE for vascular tissue engineering. J. Tissue Eng. Regen. Med. 2017;11(6):1701–1709. doi: 10.1002/term.2064. [DOI] [PubMed] [Google Scholar]
- 7.Niknejad H, Yazdanpanah G, Kakavand M. Extract of fetal membrane would inhibit thrombosis and hemolysis. Med. Hypotheses. 2015;85:197–202. doi: 10.1016/j.mehy.2015.04.030. [DOI] [PubMed] [Google Scholar]
- 8.Hori J, Wang M, Kamiya K, Takahashi H, Sakuragawa N. Immunological characteristics of amniotic epithelium. Cornea. 2006;25:53–58. doi: 10.1097/01.ico.0000247214.31757.5c. [DOI] [PubMed] [Google Scholar]
- 9.Hao Y, Ma DHK, Hwang DG, Kim WS, Zhang F. Identification of Antiangiogenic and Antiinflammatory Proteins in Human Amniotic Membrane. Cornea. 2000;19:348–352. doi: 10.1097/00003226-200005000-00018. [DOI] [PubMed] [Google Scholar]
- 10.Niknejad H, Paeini-Vayghan G, Tehrani FA, Khayat-Khoei M, Peirovi H. Side dependent effects of the human amnion on angiogenesis. Placenta. 2013;34:340–345. doi: 10.1016/j.placenta.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 11.Yazdanpanah G, Paeini-Vayghan G, Asadi S, Niknejad H. The effects of cryopreservation on angiogenesis modulation activity of human amniotic membrane. Cryobiology. 2015;71:413–418. doi: 10.1016/j.cryobiol.2015.09.008. [DOI] [PubMed] [Google Scholar]
- 12.Niknejad H, Yazdanpanah G. Anticancer effects of human amniotic membrane and its epithelial cells. Med. Hypotheses. 2014;82:488–489. doi: 10.1016/j.mehy.2014.01.034. [DOI] [PubMed] [Google Scholar]
- 13.Talmi YP, Sigler L, Inge E, Finkelstein Y, Zohar Y. Antibacterial properties of human amniotic membranes. Placenta. 1991;12:285–288. doi: 10.1016/0143-4004(91)90010-D. [DOI] [PubMed] [Google Scholar]
- 14.Deihim T, Yazdanpanah G, Niknejad H. Different Light Transmittance of Placental and Reflected Regions of Human Amniotic Membrane That Could Be Crucial for Corneal Tissue Engineering. Cornea. 2016;35(7):997–1003. doi: 10.1097/ICO.0000000000000867. [DOI] [PubMed] [Google Scholar]
- 15.Horne AW, Stock SJ, King AE. Innate immunity and disorders of the female reproductive tract. Reproduction. 2008;135:739–749. doi: 10.1530/REP-07-0564. [DOI] [PubMed] [Google Scholar]
- 16.King AE, Kelly RW, Sallenave JM, Bocking AD, Challis JRG. Innate Immune Defences in the Human Uterus during Pregnancy. Placenta. 2007;28:1099–1106. doi: 10.1016/j.placenta.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 17.Espinoza J, et al. Antimicrobial peptides in amniotic fluid: defensins, calprotectin and bacterial/permeability-increasing protein in patients with microbial invasion of the amniotic cavity, intra-amniotic inflammation, preterm labor and premature rupture of membranes. J Matern Fetal Neonatal Med. 2003;13:2–21. doi: 10.1080/jmf.13.1.2.21. [DOI] [PubMed] [Google Scholar]
- 18.Goldenberg RL, Hauth JC, Andrews WW. Intrauterine infection and preterm delivery. N. Engl. J. Med. 2000;342:1500–1507. doi: 10.1056/NEJM200005183422007. [DOI] [PubMed] [Google Scholar]
- 19.King AE, Fleming DC, Critchley HOD, Kelly R. W. regelatin of natural antibiotic expression by inflammatory mediators and mimics of infection in human endometrial epithelial cells. Mol Human Reproduction. 2002;8:341–349. doi: 10.1093/molehr/8.4.341. [DOI] [PubMed] [Google Scholar]
- 20.King AE, et al. Expression of Natural Antimicrobials by Human Placenta and Fetal Membranes. Placenta. 2007;28:161–169. doi: 10.1016/j.placenta.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 21.Ganz T. Defensins: antimicrobial peptides of innate immunity. Nat Rev Immunol. 2003;3:710–720. doi: 10.1038/nri1180. [DOI] [PubMed] [Google Scholar]
- 22.Niknejad H, Yazdanpanah G, Ahmadiani A. Induction of apoptosis, stimulation of cell-cycle arrest and inhibition of angiogenesis make human amnion-derived cells promising sources for cell therapy of cancer. Cell and Tissue Research. 2016;363(3):599–608. doi: 10.1007/s00441-016-2364-3. [DOI] [PubMed] [Google Scholar]
- 23.Buhimschi IA, et al. The novel antimicrobial peptide β3-defensin is produced by the amnion: A possible role of the fetal membranes in innate immunity of the amniotic cavity. Am. J. Obstet. Gynecol. 2004;191:1678–1687. doi: 10.1016/j.ajog.2004.03.081. [DOI] [PubMed] [Google Scholar]
- 24.Sallenave JM. Antimicrobial activity of antiproteinases. Biochem Soc. 2002;30:111–115. doi: 10.1042/bst0300111. [DOI] [PubMed] [Google Scholar]
- 25.King AE, Critchley HOD, Sallenave JM, Kelly RW. Elafin in Human Endometrium: An Antiprotease and Antimicrobial Molecule Expressed during Menstruation. JCEM. 2003;88:4426–4431. doi: 10.1210/jc.2003-030239. [DOI] [PubMed] [Google Scholar]
- 26.Stock SJ, Kelly RW, Riley SC, Calder AA. Natural antimicrobial production by the amnion. Am. J. Obstet. Gynecol. 2007;196:255.e251–255.e256. doi: 10.1016/j.ajog.2006.10.908. [DOI] [PubMed] [Google Scholar]
- 27.Ardizzoni A, et al. Influence of hyaluronic acid on bacterial and fungal species, including clinically relevant opportunistic pathogens. J Mater Sci: Mater Med. 2011;22:2329–2338. doi: 10.1007/s10856-011-4408-2. [DOI] [PubMed] [Google Scholar]
- 28.Kanyshkova TG, Buneva VN, Nevinsky GA. Lactoferrin and Its biological functions. Biochemistry. 2001;66:1–7. doi: 10.1023/a:1002817226110. [DOI] [PubMed] [Google Scholar]
- 29.Clinical Laboratory Standards Institute. (Clinical and Laboratory Standards Institute Wayne^ ePennsylvania Pennsylvania, 2006).
- 30.Niknejad H, Deihim T, Peirovi H, Abolghasemi H. Serum-free cryopreservation of human amniotic epithelial cells before and after isolation from their natural scaffold. Cryobiology. 2013;67:56–63. doi: 10.1016/j.cryobiol.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 31.Kjaergaarda N, et al. Antibacterial properties of human amnion and chorion in vitro. Eur J Obstet Gynaecol Reprod Biol. 2001;94:224–229. doi: 10.1016/S0301-2115(00)00345-6. [DOI] [PubMed] [Google Scholar]
- 32.Tehrani FA, Ahmadiani A, Niknejad H. The effects of preservation procedures on antibacterial property of amniotic membrane. Cryobiology. 2013;67:293–298. doi: 10.1016/j.cryobiol.2013.08.010. [DOI] [PubMed] [Google Scholar]
- 33.Bellemare A, Vernoux N, Morisset D, Bourbonnais Y. Human Pre-Elafin Inhibits a Pseudomonas aeruginosa-Secreted Peptidase and Prevents Its Proliferation in Complex Media. Antimicrob. Agents Chemother. 2008;52:433–490. doi: 10.1128/AAC.00585-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wiesner J, Vilcinskas A. Antimicrobial peptides: the ancient arm of the human immune system. Virulence. 2010;1:440–464. doi: 10.4161/viru.1.5.12983. [DOI] [PubMed] [Google Scholar]
- 35.Modaresifar K, et al. The effect of cryopreservation on anti-cancer activity of human amniotic membrane. Cryobiology. 2017;74:61–67. doi: 10.1016/j.cryobiol.2016.12.001. [DOI] [PubMed] [Google Scholar]
- 36.Niknejad H, et al. Inhibition of HSP90 could be possible mechanism for anti-cancer property of amniotic membrane. Med. Hypotheses. 2013;81:862–865. doi: 10.1016/j.mehy.2013.08.018. [DOI] [PubMed] [Google Scholar]
- 37.Wang X, Xie J, Tan L, Huo J, Xie H. Epithelium of human fresh amniotic membrane has antimicrobial effects in vitro. Afr. J. Microbiol. Res. 2012;6:4533–4537. [Google Scholar]
- 38.Gupta N, et al. Mesenchymal stem cells enhance survival and bacterial clearance in murine Escherichia coli pneumonia. Thorax. 2012;67:533–539. doi: 10.1136/thoraxjnl-2011-201176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sung, D. K. et al. Antibacterial effect of mesenchymal stem cells against Escherichia coli is mediated by secretion of beta-defensin-2 via toll like receptor 4 signaling. Cellular Microbiology (2015). [DOI] [PMC free article] [PubMed]
- 40.Krasnodembskaya A, et al. Antibacterial Effect of Human Mesenchymal Stem Cells Is Mediated in Part from Secretion of the Antimicrobial Peptide LL-37. Stem Cells. 2010;28:2229–2238. doi: 10.1002/stem.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.