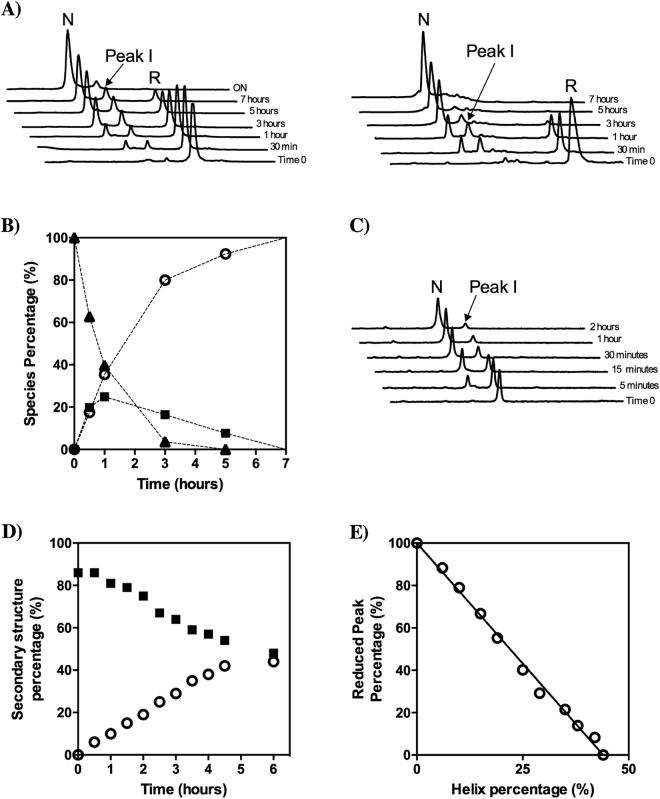
Figure 4.
Inner disulfide bond species represents a folding intermediate and displays secondary structure features linked with the native conformation. (A) hCox17 was allowed to re-oxidize after DTT removal at pH 7 (left) and pH 8.4 (right). At defined time points, the reaction was quenched and analyzed by HPLC. (B) RP-HPLC peak areas, from the refolding reaction at pH 8.4, are plotted as function of time. Reduced (solid triangles), intermediate (solid squares) and native (open circles) species. (C) Acid-trapped intermediate I was purified by RP-HPLC, freeze-dried, and dissolved in Tris-HCl (pH 8.4) to reinitiate the folding reaction. Folding reaction was subsequently quenched with acid at different time points and re-analyzed by RP-HPLC. (D) Reduced hCox17 was allowed to air oxidize at pH 8.4 inside a sealed FTIR spectrometer cuvette and the spectra recorded at specific refolding times. The relative contributions of random coil (solid squares) and α-helix (open circles) conformations to the global amide I spectra are plotted as a function of time. (E) Duplicates from the FTIR assay at pH 8.4 were acid-quenched and the different species present in the reaction analyzed by RP-HPLC as indicated above. The area of the reduced specie peak is plotted versus the α-helix content as deduced by FTIR.