Abstract
The utilisation of nanoparticles as the means of targeted delivery of therapeutics and/or imaging agents could greatly enhance the specific transport of biologically active payloads to target tissues while avoiding or reducing undesired side-effects. To allow for this to become a reality, the question of potential toxicological effects needs to be addressed. In the present investigation, a cationic liposome with prospective for medical applications was constructed and thoroughly assessed for any material-induced hepatic adverse effects in vivo − in healthy and alcoholic hepatic disease models and in vitro − (HepG2 cells). The data demonstrated that intravenous injection of liposomes did not cause any significant in vivo hepatic toxicity (inflammation, alterations in blood parameters, anti-oxidant depletion, acute phase response and histopathology) at doses of 200 μg per mouse in either healthy or chronically alcohol fed mice. Additionally, the in vitro material-induced adverse effects (cytotoxicity, inflammation or albumin secretion) were all also minimal. The data from this study demonstrated that the intravenous injection of cationic liposomes does not cause hepatic toxicity. This investigation is important as it investigates the toxicity of a nano-sized material in a model of alcoholic hepatic disease in vitro and in vivo. This is an area of research in the field of nanotoxicology that is currently almost entirely overlooked.
Keywords: Toxicology, Nanoparticles, Pharmaceutical science
1. Introduction
Liposomes are biodegradable spherical vesicles composed of an aqueous core surrounded by a phospholipid bilayer. These vesicles have been utilised as nano-sized delivery vehicles for drugs, genetic material or imaging agents (Li et al., 2015; Narayanaswamy et al., 2016). The encapsulation of relevant pharmaceutical active compounds into liposomes can control the release thus reducing systemic toxicity by minimizing dosage requirements (Bozzuto and Molinar, 2015). In addition, liposomes can help to overcome biological barriers, which is necessary to deliver the drug and exert the desired pharmacological effect. Due to their small size, charge and the possibility for modification and inclusion of targeting moieties, liposomes are ideal candidates for improved targeting and delivery efficiency (Scott et al., 2008).
Nanomedicine is defined as the application and utilisation of nanotechnology to medicine and human health, in particular with regards to the diagnosis and treatment of disease. Over the last two decades a host of various nanomaterials (NMs) with a wide range of physicochemical characteristics have been put forward as having desirable properties (optical, electronic, magnetic and biological attributes) for medical applications. Despite numerous promising new medical functions in nanomedicine, the safety concern for NMs is a key determinant factor for their clinical applications. It is imperative to establish structure-activity relationships and implement risk reduction strategies for all materials proposed for medical applications. In all reality, the same physicochemical characteristics that make NMs desirable for medical applications might contribute to their potential adverse effects (particle size and surface properties can largely influence the bioavailability, transport, biotransformation and cellular uptake) (Nel et al., 2013; Zhu et al., 2012). The importance of in vivo toxicological testing is further underlined as following bodily distribution, NMs can undergo metabolic change and/or protein coating/binding which could result in the formation of a material that in all essence is very different with regards to how it interacts with its biological surroundings in comparison the same material in vitro (Kermanizadeh et al., 2015; Sareemaspun et al., 2008).
It has been demonstrated that the liver also has significance with regards to nano-sized material accumulation and toxicity as it has been shown to preferentially accumulate large quantities of these NMs following intravenous (IV) exposure compared to other organs (Geraets et al., 2014; Kermanizadeh et al., 2015; Lipka et al., 2010; Sadauskas et al., 2009) and alongside the kidneys might be responsible for the clearance of NMs from the blood (Geiser and Kreyling, 2010; Semmler-Behnke et al., 2008). Similar to other NMs, the IV administration of non-functionalised liposomes also results in large quantities of the materials accumulating in the liver (Belmadi et al., 2016; Dicheva et al., 2016; Knudsen et al., 2014a). It is understood that global alcohol abuse was the seventh leading cause of death in 2015, with 5.2% of the mortality worldwide attributed to consumption of alcoholic beverages (Fulman et al., 2017). Additionally, the excessive consumption of alcohol is a leading cause of chronic liver disease, which results in a spectrum of disorders that range from simple fatty liver to more severe forms of liver injury such as alcoholic hepatitis, cirrhosis, and hepatocellular carcinoma affecting a large percentage of the general population which may or may not present clinical manifestations of disease (Rocco et al., 2014; Zhu et al., 2014). The liver is the principal organ for metabolising ethanol and thus considered as a major target of the associate harmful effects (Bertola et al., 2013).
We have previously reported the manufacture and efficiency of an integrin-modified positively charged liposome with great targeting potential for activated endothelial cells found in inflammatory regions of injured vasculature (Kermanizadeh et al., 2017b). However, before any consideration for the utilisation of the liposomes in medical settings, it is critical that the potential toxicological and hazardous side-effects are thoroughly investigated. This is of absolute importance with regards to hepatic toxicity as IV exposure is the principal mode of the administration for these nanocarriers. In the present study, a wide range of end-points (inflammation, alterations of bio-markers in blood, anti-oxidant depletion, acute phase response and histopathology) linked to hepatic toxicity following liposome exposure was investigated in vitro and in vivo both in healthy and in alcohol disease models. Importantly, this is the one of the first studies of its kind to consider or investigate nano-sized material-induced hepatic health effects in a model which is representative of susceptible groups of the population; i.e. individuals with pre-existing liver disease. This is an area of research in the field of nanotoxicology that is almost entirely overlooked.
2. Materials and methods
2.1. Synthesis of the liposomes
The lipids 1,2-dioleoyl-sn-glycero-3-phoshocholine (DOPC) (Avanti Polar Lipids Inc., USA), N-[1-(2,3-dioleoyloxy)propyl]-N,N,N-trimethylammonium methyl-sulfate (DOTAP) (Avanti Polar Lipids Inc., USA) and 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE)-Atto 655 (Thermo Fisher Scientific, UK) (molar ratio − 9:1:0.002) were dissolved in chloroform and thoroughly mixed in a glass vial. The solution was allowed to dry in order for a lipid film to form on the glass and to ensure the complete evaporation of the solvent. The liposomes were rehydrated by the addition of 1 x phosphate buffered saline (PBS), for a final lipid concentration of 1 mg/ml. The mixture was incubated overnight at room temperature. The following day, the liposomes were subjected to ten freeze-thaw cycles to minimize multilamellarity by immersion in liquid nitrogen followed by thawing in a 40 °C water bath. The liposomes were then sequentially extruded through two stacked polycarbonate filters with pore sizes of 50 nm (Mini-extruder − Avanti Polar Lipids Inc., USA) and stored at 4 °C until use.
2.2. Characterisation of the liposomes
The hydrodynamic size distribution (Nanosight LM20, UK), stability and surface charge of the liposomes (Zetasizer ZS, Malvern, UK) dispersed in filtered water, PBS or complete cell culture medium as well as endotoxin content (LAL Pyrogent™ Plus assay, Lonza, Switzerland) has been reported previously (Kermanizadeh et al., 2017b). A simplified version of the data following of the unonjugated liposomes in PBS and hepatocyte complete medium is presented below.
2.3. Animals, diets and liposome treatments
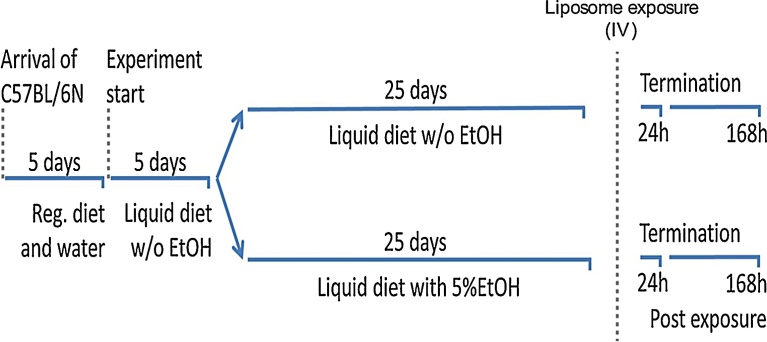
Female C57BL/6 N mice (8 weeks, approximately 20 g) were obtained from Taconic, Denmark. The mice were housed in polypropylene cages at controlled environmental conditions as previously described (Kermanizadeh et al., 2017b). The mice were fed on a regular mouse chow (Altromin 1324) for a period of 5 days before being transferred to an all liquid diet (Lieber-DeCarli 82 rodent liquid diet, control, Bio-Serv, USA) for a further 5 days. The mice were then divided into two groups with one receiving the same liquid diet while the other group were fed an all liquid diet supplemented with 5% ethanol (Lieber-DeCarli 82 rodent liquid diet, ethanol, Bio-Serv, USA) for 25 days (Fig. 1).
Fig. 1.
The diagrammatic representation of the feeding schedule.
Each animal received 20 ml of the liquid diet per day (fed every morning at the same time). The nutritional information of the liquid diets is provided in Table 1. The mice had free access to water through the feeding and liposome exposure period. The mice were weighed every 5 days during the feeding procedure as well as on the day on which they were sacrificed. Following the feeding period, the mice were anesthetized with isoflurane and exposed to 200 μg (100 μl) of liposomes or by a single injection in the lateral tail (Table 2). The relatively large dose was selected to account for a worst-case scenario in terms of potential acute toxicity. The 168 h liposome exposure animals received the same ethanol or the control liquid diet post material treatment. Following a 24 or 168 h exposure period, the mice were anaesthetized (200 μl of ZFR cocktail − Zoletil 250 mg/ml, Rompun 20 mg/ml and Fentanyl 50 μg/ml) and blood was collected form the heart before the mice were sacrificed by exsanguination. The caudate lobe (glutathione measurements) and the left lateral lobe (inflammation) of the liver were snap frozen in liquid nitrogen and stored at −80 °C. Finally, the right medial lobe was fixed in a 4% formaldehyde solution for histological analysis.
Table 1.
The nutritional profile of the two Lieber-Decarli 82 diets utilised in the feeding schedule (Kcal/litre).
| Component | Control | Ethanol |
|---|---|---|
| Protein | 151 | 151 |
| Fat | 359 | 359 |
| Carbohydrate | 490 | 135 |
| Ethanol + maltose dextrin | 0 | 355 |
Table 2.
The experimental design of the feeding and treatment groups.
| Diet | Treatment | Time point | Number of animals |
|---|---|---|---|
| Control | PBS | 24 h | 5 |
| Ethanol | PBS | 24 h | 5 |
| Control | PBS | 168 h | 5 |
| Ethanol | PBS | 168 h | 5 |
| Control | liposomes | 24 h | 5 |
| Ethanol | liposomes | 24 h | 5 |
| Control | liposomes | 168 h | 5 |
| Ethanol | liposomes | 168 h | 5 |
All animal experiments were conducted under the Danish federal guidelines for use and care of laboratory animals (complied with the EC Directive 86/609/EEC) and approved by the Danish animal inspectorate (licence number: 2012-15-2934-00223).
2.4. Blood biomarkers of liver damage
Intracardial blood samples were centrifuged to separate the serum and frozen at −80 °C for the analysis of biomarkers and the acute phase response. The serum samples were thawed and centrifuged for 10 min at 2,000 g at room temperature. The supernatant was then used for the analyses of aspartate aminotransferase (AST), alanine aminotransferase (ALT), cholesterol, triglycerides and albumin concentrations, performed on a Cobas 8000 modular analyser (Roche, USA).
2.5. Serum amyloid A3
The acute phase protein serum amyloid A3 (SAA3) was measured in serum utilising a commercially available mouse SAA3 ELISA kit (Merck Millipore, Denmark) according to the manufacturer’s instructions.
2.6. Inflammatory response
The liver samples were all thawed on ice, weighed and homogenised (gentleMACS™ Dissociator − Miltenyi Biotec, Germany) in a homogenisation buffer (PBS containing 1% Triton X–100 (Sigma, UK) containing a protease inhibitor cocktail (104 mM 4-(2-aminoethyl)benzenesulfonyl fluoride hydrochloride, 80 μM of Aprotinin, 4 mM Bestatin, 1.4 mM E–64 mM, 2 mM Leupeptin and 1.5 mM Pepstatin A) (1:100) (Sigma, UK), pH 7.2, 4 °C). The samples were thoroughly mixed (20 min) before centrifugation at 10,000 g for 10 min. The supernatant was transferred to a fresh tube and centrifuged for a further 10 min period (10,000 g). The levels of interleukin (IL) 1β, IL6, IL10, chemokine (C-X-C motif) ligand 1 (CXCL1), and monocyte chemoattractant protein-1 (MCP-1)) in the supernatant were assessed by flow cytometry (Accuri C6, BD Biosciences, USA) using BD™ Cytometric Bead Array cytokine flex sets (bead based immunoassay − BD Biosciences, USA).
2.7. Liposome-induced anti-oxidant depletion
The liver samples were weighed, thawed on ice and homogenized (as described above) in 2 ml of lysis buffer (Senft et al., 2000) and incubated for 10 min before being centrifuged at 10,000 g for 5 min to generate lysates. The reduced and total glutathione was quantified in the lysate by reaction of sulfhydryl groups with the fluorescent substrate o-phthalaldehyde (Sigma, UK) using a fluorometer with an excitation wavelength of 350 nm and emission wavelength of 420 nm.
The total glutathione levels were evaluated by reducing oxidised glutathione dimers (GSSG) to glutathione by the addition of 7 μl of 10 mM sodium dithionite to all samples and incubation at room temperature for 1 h.
2.8. Histology
The liver samples were trimmed, dehydrated and embedded in paraffin on a Tissue-Tek VIP Jr. vacuum infiltration processor (Sakura, The Netherlands). The dehydration step was followed by the clearing of samples in chloroform before the sections were cut at 5 μm on a Shandon Finesse Microtome (Axlab, Denmark). The sections were stained with haematoxylin and eosin (H&E staining) before examination by light microscopy (Leica Microsystems, Germany).
2.9. In vitro ethanol and liposome treatment
The human hepatocellular carcinoma cell line (HepG2) (Sigma Aldrich, UK) was maintained in Minimum Essential Medium Eagle (MEM) with 10% fetal bovine serum (FBS), 2 mM L-glutamine, 100 U/ml Penicillin/Streptomycin and 1% non-essential amino acids (all Sigma Aldrich, UK). On occasion and when required the HepG2 cells were exposed to 50 mM of ethanol for 24 hr. The hepatocytes (104 cells per well (96 well Plates − TRP, Switzerland) in 100 μl of the cell culture medium) were exposed to the liposomes in a concentration range between 0.62–39 μg/cm2 (equivalent to 2–125 μg/ml) for a further period of 24 hr.
2.10. WST-1 cell viability assay
Cell supernatants were removed; the wells were washed twice with PBS, followed by the addition of 10 μl of the WST-1 cell proliferation reagent (Roche, USA) and 90 μl of fresh medium. The plates were then incubated for 1 h. The supernatant was transferred to a fresh plate and the absorbance measured by dual wavelength spectrophotometry at 450 nm and 630 nm using a micro-plate reader (Multiskan FC − Thermo Scientific, USA) (supernatants were transferred to fresh plates in order to decrease the potential interference of the materials during the measurement).
2.11. In vitro production of IL8 and albumin
Following liposome treatment, cell supernatants were collected and frozen at −80 °C and later used for enzyme linked immunosorbent assays (ELISA). The supernatants were centrifuged at 1,000 g and the cytokine and albumin levels determined according to the manufacturer’s instructions (human IL8 ELISA kit − Life Technologies, UK and human albumin ELISA kit − Bethyl laboratories, USA).
2.12. Statistical analysis
The data is expressed as mean ± standard error of the mean (SEM). For statistical analysis, the data was analysed by two-way full factorial ANOVA and post-hoc multiple comparisons (Tukey) with p < 0.05 as the level of statistical significance. All statistical analysis was carried out utilizing Minitab 17 and Minitab Express.
3. Results
3.1. Characterisation of the liposomes
The characterisation data demonstrated that there was a tendency for the liposomes to form small agglomerates (∼ 100 nm in PBS and ∼ 200 nm in complete medium). However, the carriers were stable for at least three months after the initial extrusion (Table 3). Additionally, no endotoxin contamination (≤ 0.25 EU/ml) was detected in the suspension of liposomes.
Table 3.
The average mode and mean hydrodynamic size and zeta potential of the liposomes dispersed in PBS and hepatocyte complete medium. Mode − the size most abundant in the measurements. Mean − the average of the size in the measurements. The data is presented as the average ± SEM (number of independent experiments).
| Mean (nm) | Mode (nm) | Zeta potential (mV) | |
|---|---|---|---|
| PBS | 117.3 ± 17 (6) | 107.1 ± 22 (6) | +21.9 |
| Complete MEM | 208.0 ± 57 (3) | 185.4 ± 33 (3) | Not tested |
3.2. In vivo data
3.2.1. Bodyweight changes in the alcohol fed and/or liposome exposed mice
There was no significant change in the body weight of ethanol vs. control fed and or liposome exposed mice (data not shown). In addition, no visible sign of discomfort was noted in any of the mice during the feeding period or following the liposome exposure.
3.2.2. Hepatic inflammation
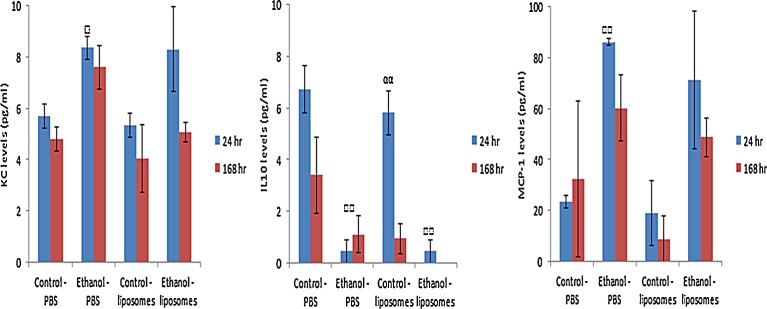
The analysis of hepatic inflammation demonstrated that there was no obvious liposome-induced effect at the time points and cytokines/chemokines investigated. However, there was a significant and fairly large decrease in the levels of anti-inflammatory IL10 in the alcohol fed mice as compared to the control liquid diet fed mice (Fig. 2b). Interestingly, a time-dependent difference was noted in the control diet fed and liposome exposed mice. However, these values were not statistically significantly different from the appropriate PBS treated controls. Furthermore, a significant increase in the levels of KC and MCP-1 was observed in the alcohol fed mice (Fig. 2a, c). The data clearly demonstrated an alcohol stimulated disturbance to the normal “immune tolerant” milieu of the healthy liver.
Fig. 2.
Cytokine levels from control and alcohol fed mice livers following intravenous exposure of 200 μg of liposomes for 24 or 168 h. The values depict mean ± SEM (n = 5), significance indicated by □ = p < 0.05 and □□ = p < 0.005, when indicative of an alcohol induced effect and αα = p < 0.005 when a time-dependent effect is exhibited.
No significant change in levels of hepatic specific IL1β or IL6 was detected for any of the treatments and time-points investigated (IL1β − 0 pg/ml for the controls, all treatments and time points; IL6 24 h − control-PBS 0.56 ± 0.4, ethanol-PBS 0.55 ± 0.42, control-liposomes 0.09 ± 0.09, ethanol-liposomes 0.32 ± 0.2 pg/ml; IL6 168 h − control-PBS 0.23 ± 0.09, ethanol-PBS 0.1 ± 0.1, control-liposomes 0.05 ± 0.03, ethanol-liposomes 0.18 ± 0.13 pg/ml).
3.2.3. Blood biomarkers of liver damage
The analysis of blood biomarkers showed some statistically significant changes which in majority were small with no discerning pattern observed. Unfortunately, haemolysis in some samples affected the AST readings and resulted in fairly large variations in these measurements. Overall the biochemical variables did not show consistent evidence of liposome-induced toxicity to the liver (with the exception of some fluctuations in the serum cholesterol levels however these were small and inconsistent between the time-points) (Table 4). As expected the alcohol consumption resulted in increased levels of AST and ALT in the blood (although not statistically significant). The AST results findings should be considered with a degree of caution because of haemolysis in some samples. Finally, time-dependent differences were noted for several variables; however, no discriminating pattern was observed between these alterations. It should be stated that previous studies also show that chronic ethanol diets only result in mild changes in blood biomarkers of liver toxicity (Bertola et al., 2013; Cohen et al., 2010; Mandrekar et al., 2011). To that end, the lack of significant response is not all that surprising.
Table 4.
Liver toxicity blood bio-markers assessed in the serum of liposome exposed mice sacrificed 24/168 h post treatment. The values depict mean ± SEM (n = 5), significance indicated by * = p < 0.05 and ** = p < 0.005, when indicative of an alcohol induced effect, ▽ = p < 0.05 and ▽▽ = p < 0.005 when a liposome-induced effect is observed. Interaction of alcohol and liposome effects signified by ♢ = p < 0.05.
| Biomarker of interest | Control diet − PBS | Ethanol diet − PBS | Control diet − liposomes | Ethanol diet − liposomes |
|---|---|---|---|---|
| ALT (U/l) − 24 h - 168 h |
23.52 ± 1.68 | 30 ± 2.85 | 25.44 ± 2.51 | 29.54 ± 6.37 |
| 30.26 ± 2.71 | 36.04 ± 4.83 | 24.12 ± 1.36 | 45.94 ± 9.87 | |
| AST (U/l) − 24 h ♢ - 168 h |
111 ± 23.3 | 187.3 ± 31.2 | 164.4 ± 17.1 | 118.7 ± 26.4 |
| 266.1 ± 40.4 | 178.6 ± 54.4 | 142.2 ± 37.9 | 162.3 ± 14.8 | |
| Albumin (g/l) − 24 h - 168 h |
12.38 ± 0.01 | 13.3 ± 0.33* | 12.1 ± 0.11 | 12.38 ± 0.12▽ |
| 13.64 ± 0.19 | 13.26 ± 0.31 | 12.2 ± 0.22▽▽ | 12.64 ± 0.27 | |
| Cholesterol (mmol/l) − 24 h ♢ - 168 h ♢ |
2.07 ± 0.08 | 2.08 ± 0.02 | 2.27 ± 0.06 | 1.94 ± 0.06* |
| 1.98 ± 0.03 | 2.1 ± 0.1 | 1.96 ± 0.1 | 2.53 ± 0.08**▽▽ | |
| Triglycerides (mmol/l) − 24 h - 168 h |
0.82 ± 0.14 | 0.9 ± 0.16 | 0.68 ± 0.08▽ | 0.76 ± 0.11 |
| 0.5 ± 0.03 | 0.64 ± 0.09 | 0.63 ± 0.08 | 0.67 ± 0.06 |
3.2.4. Glutathione levels in the liver
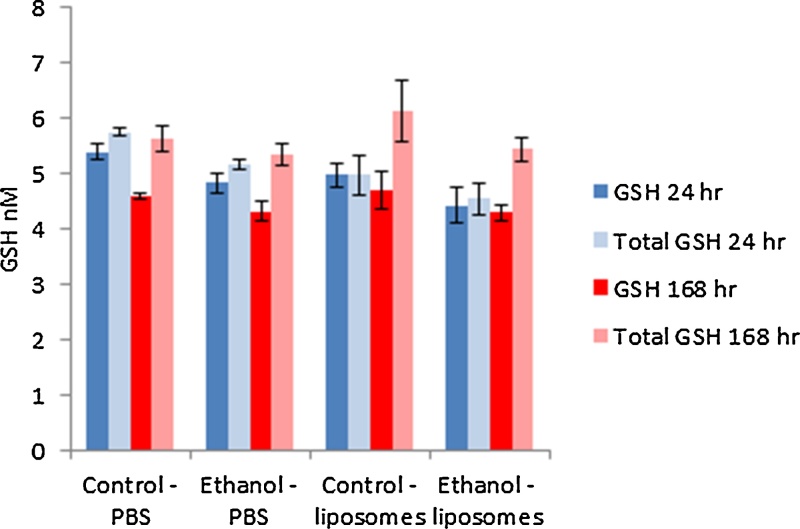
As a measure of oxidative stress, reduced and total glutathione content were quantified in the liver homogenates of the exposed mice (Fig. 3). The data demonstrated that there was no significant difference in GSH levels following either the alcohol or the liposome exposure at the two time-points investigated.
Fig. 3.
The reduced (GSH) and total glutathione (Total GSH) measured in the livers of control and ethanol fed animal exposed to 200 μg of liposomes at 24 and 168 h. The values depict mean ± SEM (n = 5).
3.2.5. SAA3 concentrations in serum
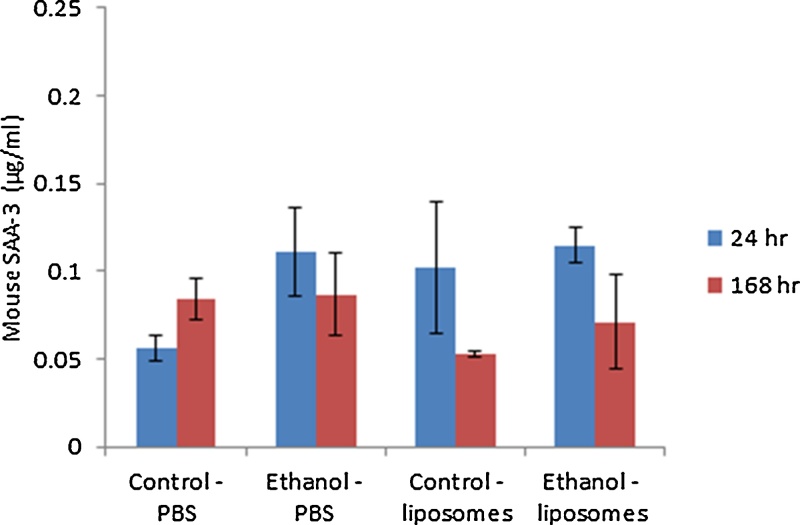
The data indicated that there was a small (non-significant) increase in SAA3 concentrations in serum of the alcohol fed animals while, no liposome-induced acute phase response was noted at either of the two time-points investigated (Fig. 4).
Fig. 4.
SAA3 measured in the serum of control and ethanol fed animal exposed to 200 μg of liposomes for 24 or 168 h. The values depict mean ± SEM (n = 5).
3.2.6. Histology
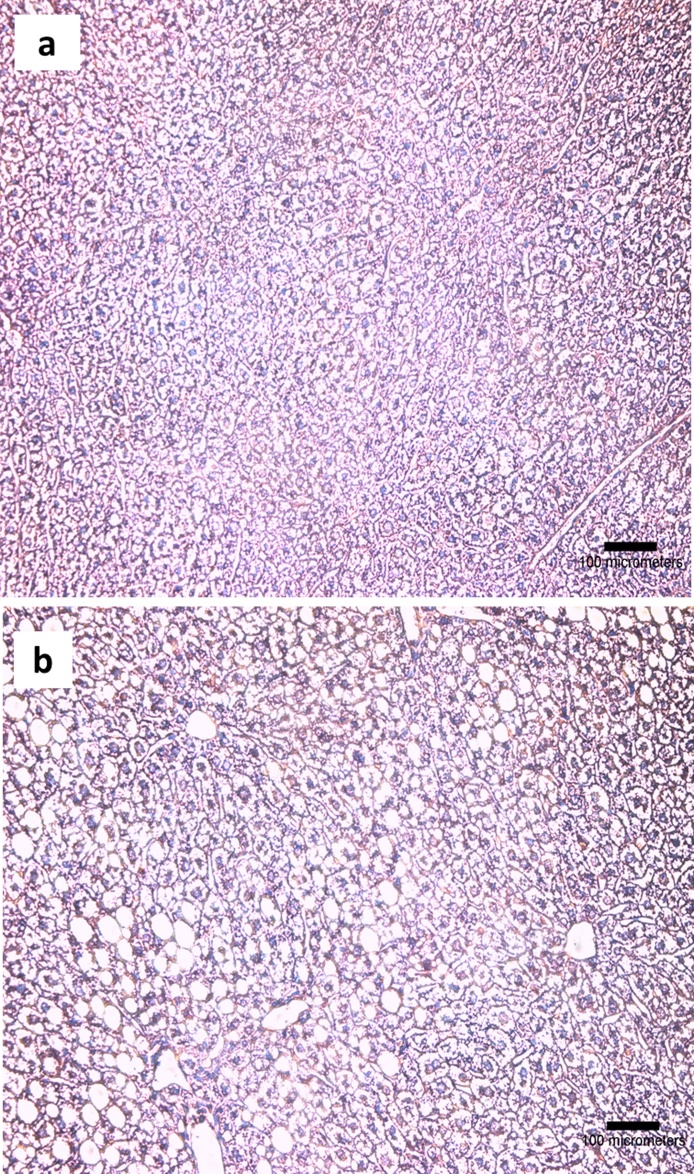
The chronic alcohol consumption resulted in hepatic injury manifested most notably as steatosis and slight necrosis (Fig. 5b). These findings were comparable between the mice scarified at 24 and 168 h after liposome or vehicle exposure (Table 5). The exposure to liposomes did not generate pathological effects in the group of mice on alcohol-free control diet or the alcohol supplemented diet (with the exception of a very few incidences of cytoplasmic degeneration in one of the animals) (Table 5).
Fig. 5.
The histopathological examination of H&E stained liver tissue from a) mice on control diet, b) mice on the alcohol supplemented diet. Foci with steatosis and slight necrosis are present in the image from alcohol exposed mice, whereas the architecture of the liver is normal in the image of the mouse from the control diet group. The images are representative for each of the groups of 3 mice investigated.
Table 5.
The histological score of liver pathology from three slides from three random animals for each treatment group ranked from 0–5 (none to severe).
| 24 h | Control diet − PBS | Ethanol diet − PBS | Control diet − liposomes | Ethanol diet − liposomes |
|---|---|---|---|---|
| Steotosis | 0, 0, 0 | 4, 4, 3 | 0, 0, 0 | 3, 3, 4 |
| Inflammation | 0, 0, 0 | 0, 0, 1 | 1, 1, 1 | 1, 0, 1 |
| Necrosis | 0, 0, 0 | 0, 1, 0 | 0, 0, 0 | 0, 0, 0 |
| 168 h | Control diet − PBS | Ethanol diet − PBS | Control diet − liposome | Ethanol diet − liposome |
| Steotosis | 0, 0, 0 | 5, 5, 5 | 0, 1, 0 | 4, 5, 5 |
| Inflammation | 0, 0, 0 | 0, 1, 0 | 1, 0, 1 | 0, 1, 1 |
| Necrosis | 0, 0, 0 | 0, 1, 1 | 0, 0, 0 | 1, 0, 0 |
3.3. In vitro data
3.3.1. Impact of liposome exposure on HepG2 cell cytotoxicity
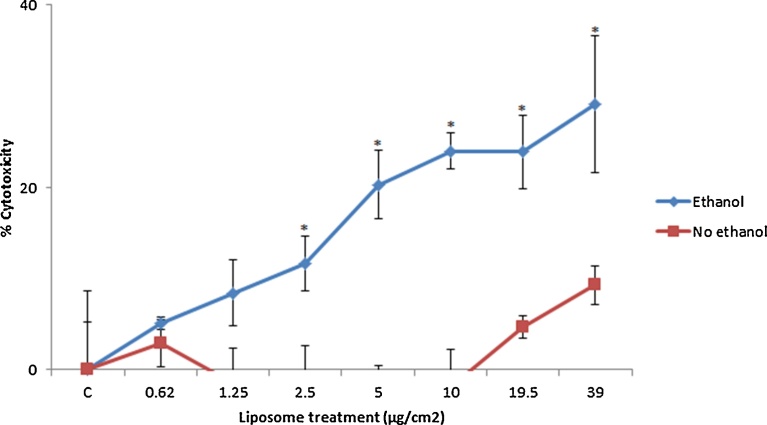
From the WST-1 data it was evident that there was very little cytotoxicity associated with liposome exposure even at concentrations up to 39 μg/cm2. That being said a significant difference could be observed between the ethanol pre-treated hepatocytes and the controls at the five highest concentrations (Fig. 6). The data showed that ethanol pre-treated cells are more susceptible to cytotoxicity by co-exposure to liposomes.
Fig. 6.
Cytotoxicity in control and ethanol (50 mM) pre-treated HepG2 cells following exposure to cationic liposomes. The cells were exposed to cell medium (control)/increasing concentrations of the liposomes for 24 h with cytotoxicity measured via WST-1 assay. The values represent mean ± SEM (n = 3 − three separate days), significance between the groups indicated by * = p < 0.05.
3.3.2. Impact of liposome exposure on HepG2 IL8 production
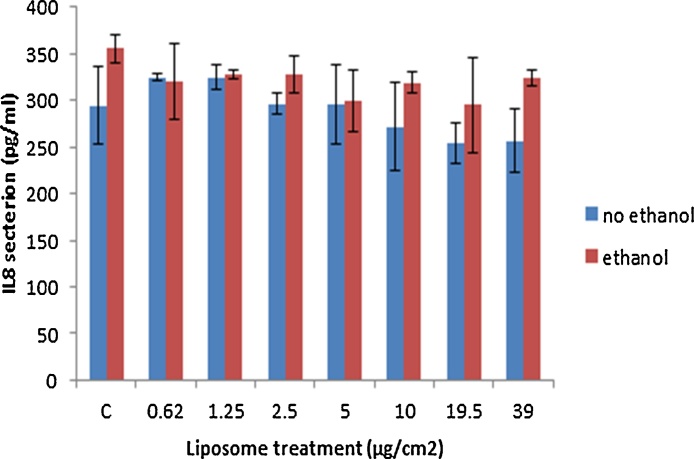
The changes in cytokine secretion as a consequence of liposome exposure was assessed in the supernatant of exposed hepatocytes and quantified via ELISA. The data demonstrated no effect of liposome exposure on the levels of the chemokine secreted from the cells. In addition, alcohol pre-treatments seemed to have no significant effect on this end-point (Fig. 7).
Fig. 7.
IL8 secretion within HepG2 cell supernatants following a 24 h exposure period to increasing concentrations of the liposomes. The values represent mean ± SEM (n = 3 − three separate days).
3.3.3. Impact of the liposome exposure on albumin levels in HepG2 cells
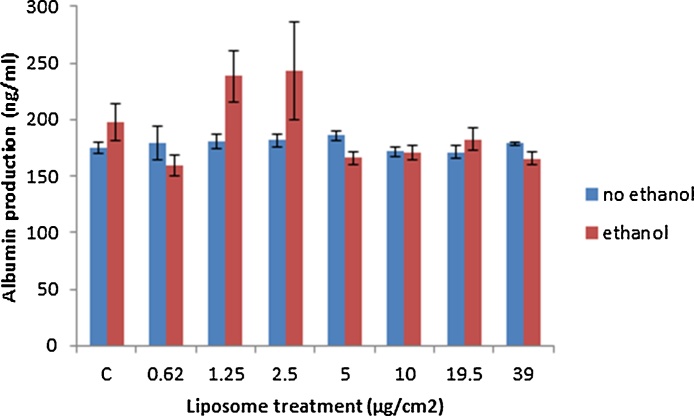
Albumin levels were measured as a marker of hepatocyte function in ethanol and/or liposome treated cells. The data showed no effect of either ethanol pre-treatment or liposome exposure on albumin secretion from the hepatocytes at the concentrations investigated (Fig. 8).
Fig. 8.
Albumin production from non and pre-ethanol treated HepG2 cells following exposure to increasing concentrations of the liposomes. The cells were exposed to medium (control) or the liposomes for 24 h. The values represent mean ± SEM (n = 3 − three separate days).
4. Discussion
In this study, we demonstrate that the cationic liposome nanocarriers, which are prime candidates for utilisation in medical applications, induced no adverse effects to hepatocytes in vitro at concentrations of up 39 μg/cm2. Additionally, IV exposure of liposomes did not cause any significant hepatic toxicity in vivo at doses of up to 200 μg per mouse either in healthy or chronically alcohol fed mice. This study is important as it sets the foundations for future in vivo exploitation of the carriers as it is one of the first studies to investigate nano-sized liposome toxicity in models of alcoholic hepatic disease in vitro and in vivo.
In this study, almost all hepatic adverse effects investigated (changes in the inflammatory profile in the liver, alteration in blood bio-markers and histopathological observations in terms of moderate fatty changes (steatosis)) were induced by the chronic alcohol feeding of animals. Similar findings have previously been reported in alcohol fed animals (albeit different feeding periods/alcohol concentrations/different combination of end-point investigated) (Anthony et al., 2010; Ki et al., 2010; Rhodes et al., 2005). However, there was no evidence that liposome exposure results in any significant adversity in the liver of healthy animals or caused intensification of disease in the alcohol fed animals at either time-point investigated. The data from this study fits very well with our previous findings in which very little toxicity (measured in terms of mitochondrial function and inflammatory response) was observed in vitro following liposome treatment of mono-cultured endothelial cells and co-cultures of endothelial cells and macrophages (Kermanizadeh et al., 2017b). It should be noted that our animal model was able to detect adverse effects of silver NM exposure on most of the measured end-points and was aggravated by prior alcohol exposure (Kermanizadeh et al., 2017a).
In concordance to the findings within this study the principal body of in vitro and in vivo investigations into toxicity associated with empty cationic liposome exposure have also demonstrated the adverse effects to be very limited and restricted to high non-physiologically relevant doses/concentrations. As some examples, the effects of cationic liposome delivery systems (∼ 100 nm DOTAP/cholesterol) were investigated in vitro in A549 and HepG2 cell lines (cytotoxicity, lipid accumulation, genotoxicity and secretion of pro-inflammatory cytokines) and in vivo (Han Wistar rats intravenously exposed to the liposomes at doses of 10, 25 or 100 mg/kg with analysis of blood bio-markers, histopathology and genotoxicity) (Knudsen et al., 2015; Roursgaard et al., 2016). The authors showed that, liposome-induced toxicity was only limited to concentrations above 100 μg/ml in A549 and HepG2 cells (Roursgaard et al., 2016). The study of the in vivo toxicity effects only demonstrated very limited effects of liposome administration − in terms of histopathology and clinical chemistry (Knudsen et al., 2015). Similarly, the repeated IV administration of the same liposome at doses of 100 mg/kg on three separate occasions over a period of a week was well tolerated and not associated with any systemic toxicity (Knudsen et al., 2014b). However, it should be stated that cationic liposome toxicity has been observed in in vitro settings with cell type being an important determinant of the adverse effects detected; i.e. macrophages are highly susceptible to cytotoxic effects of liposome exposure − in all probability due to high uptake by these professional phagocytes (Iwaoka et al., 2006; Nogueira et al., 2013; Romoren et al., 2004; Xia et al., 2008).
Alcohol abuse can have an impact not only on the incidence of disease (most prominently liver cirrhosis), but also on the progression of disorders; making alcohol-attributable disease a major factor in global burden of health corollaries (Rehm and Shield, 2013). There is extensive variation in the total alcohol consumption globally and even a wider spectrum of adverse effect associated with alcohol abuse, meaning that a large percentage of the overall population could be suffering from unregistered sub-clinical symptoms. Therefore, it is crucial that this large and vulnerable sub-group of the general population is included in any hazard and risk assessment strategy. In this study, we show that our liposomes do not have any adverse health effects in alcohol damaged liver or ethanol exposed hepatocytes as models of susceptibility to liver cirrhosis. This is one of the only two nanotoxicological studies to include this important variable in the hazard assessment of materials utilising a life-style relevant material, dose and route of exposure.
Currently, the majority of the research in the field of nanomedicine is focused on the application of NMs with relatively little attention paid to the potential toxicological consequences. As a corollary, the systematic toxicological evaluation of NMs is often not ruminated during the product development process. It is extremely important to fully consider the ramifications of the utilisation of unique exposure routes in nanomedicine that intentionally bypass the normal absorption processes which might be a cause of real concern with regards to systemic material-induced toxicity. Therefore, it is imperative that a wide range of toxicity and relatable end-points with appropriate doses is investigated for safe design and utilisation of nano-constructs for medical applications. In this study, we show the production of highly reproducible stable liposomes with very little toxic potential both in vitro and in healthy or in alcohol-exposed animals (as a representative model of individuals in general population with pre-existing hepatic medical complications).
Declarations
Author contribution statement
Ali Kermanizadeh: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Nicklas R. Jacobsen: Conceived and designed the experiments; Performed the experiments.
Martin Roursgaard: Performed the experiments.
Steffen Loft, Peter Møller: Conceived and designed the experiments; Contributed to purchase of reagents and materials.
Funding statement
This work was supported by the Danish Council of Independent Research(Medical Sciences, grant no. 12-126262) and by theDanish Centre for Nanosafety II.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors are grateful to colleagues at the National Research Centre for Working Environment (Michael Guldbrandsen, Eva Terrida and Natascha Synnøve Olsen) and University of Copenhagen.
References
- Anthony B., Vinci-Booher S., Wetherill L., Ward R., Goodlett C., Zhou F.C. Alcohol-induced facial dysmorphology in C57BL/6 mouse models of fetal alcohol spectrum disorder. Alcohol. 2010;44:659–671. doi: 10.1016/j.alcohol.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belmadi N., Berchel M., Denis C., Berthe W., Sibiril Y., Le Gall T., Haelters J.P., Jaffres P.A., Montier T. Evaluation of new fluorescent lipophosphoramidates for gene transfer and biodistribution studies after systemic administration. Int. J. Mol. Sci. 2016;16:26055–26076. doi: 10.3390/ijms161125941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertola A., Mathews S., Ki S.H., Wang H., Gao B. Mouse model of chronic and binge ethanol feeding (the NIAAA model) Nat. Protoc. 2013;8:627–637. doi: 10.1038/nprot.2013.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozzuto G., Molinar A. Liposomes as nanomedical devices. Int. J. Nanomedicine. 2015;10:975–999. doi: 10.2147/IJN.S68861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J.I., Roychowdhury S., McMullen M.R., Stavitsky A.B., Nagy L.E. Complement and alcoholic liver disease: role of C1q in the pathogenesis of ethanol-induced liver injury in mice. Gastroenterology. 2010;139:664–674. doi: 10.1053/j.gastro.2010.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dicheva B.M., Seynhaeve A.L.B., Soulie T., Eggermont A.M.M., ten Hagen T.L.M., Koning G.A. Pharmacokinetics, tissue distribution and therapeutic effect of cationic thermosensitive liposomal doxorubicin upon mild hyperthermia. Pharm. Res. 2016;33:627–638. doi: 10.1007/s11095-015-1815-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulman N., GBD 2016, SDG collaborators Measuring progress and projecting attainment on the basis of past trends of the health-related sustainable development goals in 188 countries: an analysis from the global burden of disease study 2016. Lancet. 2017;390:1423–1459. doi: 10.1016/S0140-6736(17)32336-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiser M., Kreyling W.G. Deposition and biokinetics of inhaled nanoparticle. Part. Fibre Toxicol. 2010;7:2. doi: 10.1186/1743-8977-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geraets L., Oomen A.G., Krystek P., Jacobsen N.R., Wallin H., Laurentie M., Verharen H.W., Brandon E.F., de Jong W.H. Tissue distribution and elimination after oral and intravenous administration of different titanium dioxide nanoparticles in rats. Part. Fibre Toxicol. 2014;11:30. doi: 10.1186/1743-8977-11-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwaoka S., Nakamura T., Takano S., Tsuchiya S., Aramaki Y. Cationic liposome induce apoptosis through p38 Map kinase-caspase 8-Bid pathway in macrophage-like RAW264.7 cells. J. Leukoc. Biol. 2006;79:184–191. doi: 10.1189/jlb.0405181. [DOI] [PubMed] [Google Scholar]
- Kermanizadeh A., Balharry D., Wallin H., Loft S., Møller P. Nanomaterial translocation − the biokinetics, tissue accumulation, toxicity and fate of materials in secondary organs − a review. Crit. Rev. Toxicol. 2015;45:837–872. doi: 10.3109/10408444.2015.1058747. [DOI] [PubMed] [Google Scholar]
- Kermanizadeh A., Jacobsen N.R., Roursgaard M., Loft S., Møller P. Hepatic hazard assessment of silver nanoparticle exposure in healthy and chronically alcohol fed mice. Toxicol. Sci. 2017;158:176–187. doi: 10.1093/toxsci/kfx080. [DOI] [PubMed] [Google Scholar]
- Kermanizadeh A., Villadsen K., Østrem R.G., Jensen K.J., Møller P., Loft S. Integrin targeting and toxicological assessment of peptide-conjugated liposome delivery systems to activated endothelial cells. Basic Clin. Pharmacol. Toxicol. 2017;120:380–389. doi: 10.1111/bcpt.12692. [DOI] [PubMed] [Google Scholar]
- Ki S.H., Park O., Zheng M., Morales-Ibanez O., Kolls J.K., Bataller R., Gao B. Interleukin-22 treatment ameliorates alcoholic liver injury in a murine model of chronic-binge ethanol feeding: role of signal transducer and activator of transcription 3. Hepatology. 2010;52:1291–1300. doi: 10.1002/hep.23837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen K.B., Northeved H., Gjetting T., Permin A., Andresen T.L., Wegener K.M., Lam H.R., Lykkesfeldt J. Biodistribution of rhodamine B fluorescence-labeled cationic nanoparticles in rats. J. Nanopart. Res. 2014;16:2221. [Google Scholar]
- Knudsen K.B., Northeved H., Kumar P.E.K., Permin A., Andresen T.L., Larsen S., Wegener K.L., Lam H.R., Lykkesfeldt J. Differential toxicological response to positively and negatively charged nanoparticles in the rat brain. Nanotoxicology. 2014;8:764–774. doi: 10.3109/17435390.2013.829589. [DOI] [PubMed] [Google Scholar]
- Knudsen K.B., Northeved H., Kumar P.E.K., Permin A., Gjetting T., Andresen T.L., Larsen S., Wegener K.M., Lykkesfeldt J., Jantzen K., Loft S., Møller P., Roursgaard M. In vivo toxicity of cationic micelles and liposomes. Nanomed. Nanotech. Biol. Med. 2015;11:467–477. doi: 10.1016/j.nano.2014.08.004. [DOI] [PubMed] [Google Scholar]
- Li F., Jin L., He L., Deng Y., He N.Y. Nanoparticles applied for therapy and diagnosis in common diseases. Sci. Adv. Mater. 2015;7:2103–2122. [Google Scholar]
- Lipka J., Semmler-Behnke M., Sperling R.A., Wenk A., Takenaka S., Schleh C., Kissel T., Parak W.J., Kreyling W.G. Biodistribution of PEG-modified gold nanoparticles following intratracheal instillation and intravenous injection. Biomaterials. 2010;31:6574–6581. doi: 10.1016/j.biomaterials.2010.05.009. [DOI] [PubMed] [Google Scholar]
- Mandrekar P., Ambade A., Lim A., Szabo G., Catalano D. An essential role for monocyte chemoattractant protein-1 in alcoholic liver injury: regulation of proinflammatory cytokines and hepatic steatosis in mice. Hepatology. 2011;54:2185–2197. doi: 10.1002/hep.24599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanaswamy R., Wang T., Torchilin V.P. Improving peptide applications using nanotechnology. Curr. Top. Med. Chem. 2016;16:253–270. doi: 10.2174/1568026615666150817100338. [DOI] [PubMed] [Google Scholar]
- Nel A., Xia T., Meng H., Wang H., Wang X., Lin S., Ji Z., Zhang H. Nanomaterial toxicity testing in the 21st century: use of a predictive toxicological approach and high-throughput screening. Acc. Chem. Res. 2013;46:607–621. doi: 10.1021/ar300022h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogueira D.R., Morán M.C., Mitjans M., Martínez V., Pérez L., Vinardell M.P. New cationic nanovesicular systems containing lysine-based surfactants for topical administration: Toxicity assessment using representative skin cell lines. Eur. J. Pharm. Biopharm. 2013;83:33–43. doi: 10.1016/j.ejpb.2012.09.007. [DOI] [PubMed] [Google Scholar]
- Rehm J., Shield K.D. Alcohol and Mortality − Global alcohol-attributable deaths from cancer, liver cirrhosis, and injury in 2010. Alcohol Res. 2013;35:174–183. [PMC free article] [PubMed] [Google Scholar]
- Rhodes J.S., Best K., Belknap J.K., Finn D.A., Crabbe J.C. Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice. Physiol. Behav. 2005;84:53–63. doi: 10.1016/j.physbeh.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Rocco A., Compare D., Angrisani D., Zamparelli M.S., Nardone G. Alcoholic disease: liver and beyond. World J. Gastroenterol. 2014;20:14652–14659. doi: 10.3748/wjg.v20.i40.14652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romoren K., Thu B.J., Bols N.C., Evensen O. Transfection efficiency and cytotoxicity of cationic liposomes in Salmonid cell lines of hepatocyte and macrophage origin. Biochim. Biophys. Acta − Biomembranes. 2004;1663:127–134. doi: 10.1016/j.bbamem.2004.02.007. [DOI] [PubMed] [Google Scholar]
- Roursgaard M., Knudsen K.B., Northeved H., Persson M., Christensen T., Kumar P.E.K., Permin A., Andresen T.L., Gjetting T., Lykkesfeldt J., Vesterdal L.K., Loft S., Møller P. In vitro toxicity of cationic micelles and liposomes in cultured human hepatocyte (HepG2) and lung epithelial (A549) cell lines. Toxicol. In Vitro. 2016;36:164–171. doi: 10.1016/j.tiv.2016.08.002. [DOI] [PubMed] [Google Scholar]
- Sadauskas E., Jacobson N.R., Danscher G., Soltenberg M., Larsen A., Kreyling W., Wallin H. Bio-distribution of gold nanoparticles in mouse lung following intratracheal instillation. Chem. Cent. J. 2009;3:16–23. doi: 10.1186/1752-153X-3-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sareemaspun A., Rojanathanes R., Wiwanitkit V. Effect of gold nanoparticle on renal cell: an important for exposure risk. Renal Failure. 2008;30:323–325. doi: 10.1080/08860220701860914. [DOI] [PubMed] [Google Scholar]
- Scott R.C., Crabbe D., Krynska B., Ansari R., Kiani M.F. Aiming for the heart: targeted delivery of drugs to diseased cardiac tissue. Expert Opin. Drug Deliv. 2008;5:459–470. doi: 10.1517/17425247.5.4.459. [DOI] [PubMed] [Google Scholar]
- Semmler-Behnke M., Kreyling W.G., Lipka J., Fertsch S., Wenk A., Takeneka S., Schmid G., Brandau W. Bio-distribution of 1.4 and 18 nm gold particles in rats. Small. 2008;12:2108–2111. doi: 10.1002/smll.200800922. [DOI] [PubMed] [Google Scholar]
- Senft A.P., Dalton T.P., Shertzer H.G. Determining glutathione and glutathione disulfide using the fluorescence probe o-phthalaldehyde. Anal. Biochem. 2000;280:80–86. doi: 10.1006/abio.2000.4498. [DOI] [PubMed] [Google Scholar]
- Xia T., Kovochich M., Liong M., Zink J.I., Nel A.E. Cationic polystyrene nanosphere toxicity depends on cell-specific endocytic and mitochondrial injury pathways. ACS Nano. 2008;2:85–96. doi: 10.1021/nn700256c. [DOI] [PubMed] [Google Scholar]
- Zhu S., Ma L., Wu Y., Ye X., Zhang T., Zhang Q., Rasoul L.M., Liu Y., Guo M., Zhou B., Ren G., Li D. FGF21 treatment ameliorates alcoholic fatty liver through activation of AMPK-SIRT1 pathway. Acta Biochim. Biophys. Sin. 2014;46:1041–1048. doi: 10.1093/abbs/gmu097. [DOI] [PubMed] [Google Scholar]
- Zhu Y., Eaton J.W., Li C. Titanium dioxide (TiO2) nanoparticles preferentially induce cell death in transformed cells in a Bak/Bax-independent fashion. Plos One. 2012;7 doi: 10.1371/journal.pone.0050607. [DOI] [PMC free article] [PubMed] [Google Scholar]