Figure 12.
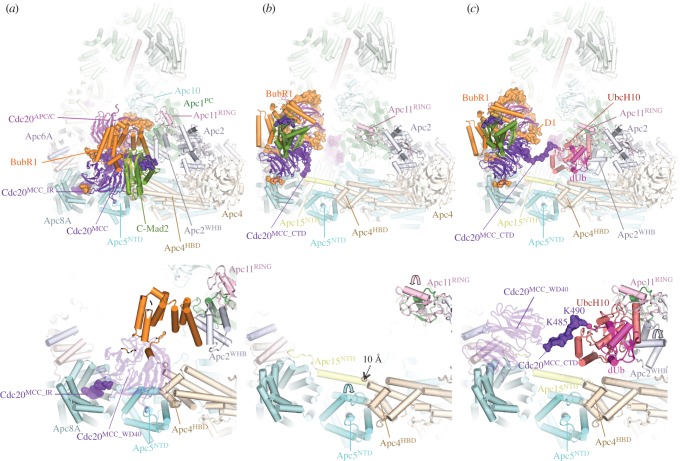
APC/CMCC adopts open and closed states that allows for reciprocal control by the MCC. (a) In APC/CMCC-closed, the MCC inhibits both substrate (for example, securin and cyclin B) and UbcH10 recognition. Upper panel: overall APC/CMCC structure. Lower panel: shows how binding of the MCC in APC/CMCC-closed causes an upward movement of Apc5NTD and Apc4HBD (compare with b) and concomitant disordering of the N-terminal helix of Apc15 (Apc15NTH). (b) In APC/CMCC-open, the catalytic module is exposed, Apc5NTD rotates, Apc4HBD translates down by 10 Å, and Apc15NTH is ordered. Movements of Apc4HBD, Apc5NTD and Apc11RING domain are indicated with arrows. (c) In the APC/CMCC-UbcH10 complex, APC/CMCC adopts the open conformation with Apc15NTH ordered and UbcH10 docking to its canonical position on Apc11RING and Apc2WHB. The C-terminal tail of Cdc20MCC engages the catalytic site of UbcH10 for auto-ubiquitination of Lys485 and Lys490. In APC/CMCC-closed, the C-terminal IR tail of Cdc20MCC engages the C-box binding site of Apc8A. From Alfieri et al. [92].