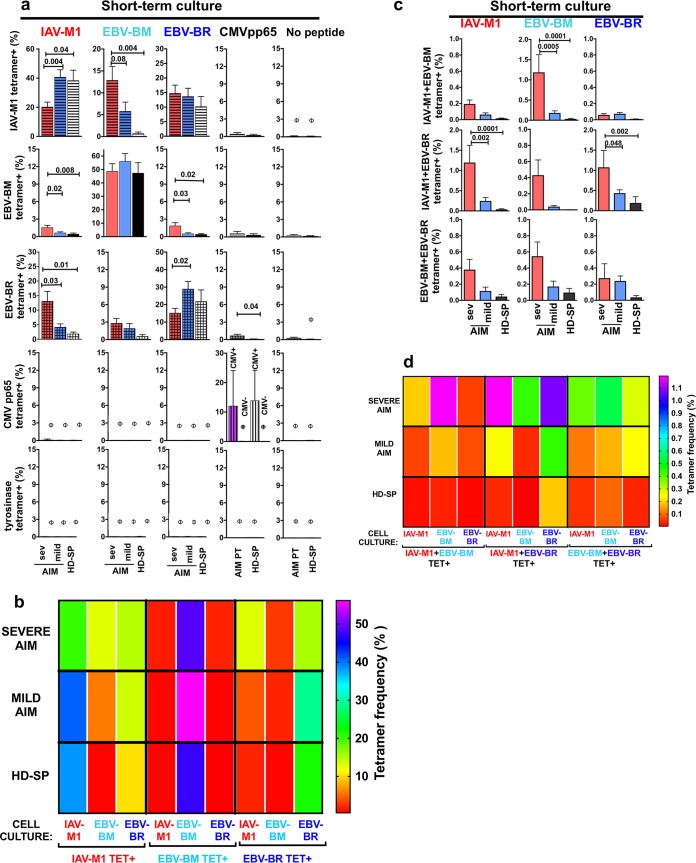
FIG 6 .
Severe-AIM patients have the greatest proliferation of IAV/EBV cross-reactive CD8 T cells in short-term in vitro cultures. In addition, each patient group maintains unique identifiable cognate and cross-reactive CD8 T-cell proliferation profiles upon peptide stimulation in short-term in vitro cultures, which are representative of their in vivo CD8 T-cell repertoires. The proliferation of IAV-M1-, EBV-BM-, and EBV-BR-specific cells in each culture was determined by costaining with pairs of tetramers (7 to 18 donors per group). In each culture, we determined the tetramer frequency of cognate (same as the culture) (a and b) and cross-reactive cells that were either single tetramer+ (a and b) or double tetramer+ (c and d). (a) Increased expansion of single tetramer+ cross-reactive cells in EBV-BM- and IAV-M1-stimulated cultures of cells from severe-AIM patients. There was significantly increased expansion of IAV-M1 tetramer+ cells in BM-stimulated cultures of cells from severe-AIM patients versus those from mild-AIM patients or HD-SP. Within the severe-AIM group, the cognate IAV-M1 tet+ frequency is lower than that in the mild-AIM group; instead, this group has a significantly increased frequency of cross-reactive IAV-M1 tet+ cells that grew in response to EBV-BM stimulation. Within the severe-AIM group, the cognate EBV-BR tet+ frequency is lower than that in mild-AIM patients; instead, this group has a significantly increased cross-reactive EBV-BR tet+ frequency in the IAV-M1-stimulated culture. Control cultures included short-term cultures with CMV pp65 or tyrosinase peptide or T2 cells (antigen-presenting cells) alone without a peptide, where the double tetramer frequencies were ≤0.1%, as indicated by the symbol φ. (b) Each donor group has unique profiles of cognate and cross-reactive IAV/EBV-specific CD8 T-cell proliferation that are highly significantly different from each other, as demonstrated in a heat map display with multivariate analyses. (c) Increased expansion of IAV/EBV double tetramer+ cross-reactive cells in severe (sev)-AIM patients. There were significantly higher frequencies of IAV-M1+EBV-BM tet+ cells in an EBV-BM-stimulated culture and IAV-M1+EBV-BR tet+ cells in both IAV-M1- and EBV-BR-stimulated cultures of cells from severe-AIM patients than in those of cells from mild-AIM patients or HD-SP. (The mean of all double tetramer+ frequencies pairing IAV-M1, EBV-BM, and EBV-BR tet+ with control tyrosinase tet+ was <0.1; the mean of all double tetramer+ frequencies in control CMV pp65-, tyrosinase-, and no-peptide-stimulated cultures was <0.1.) (d) Severe-AIM patients have a unique profile of double tetramer+ cross-reactive IAV/EBV-specific CD8 T-cell proliferation, as demonstrated in a heat map display with multivariate analyses. The Student t test was used to compare two groups, one-way ANOVA with Sidak’s multiple-comparison test was used to compare more than two groups, and multivariate analyses were done by two-way ANOVA with Tukey’s multiple-comparison test with adjustment for multiple comparisons. Details of the highly significant but complex multivariate statistical analyses for the heat maps (b and d) are shown in Fig. S5.