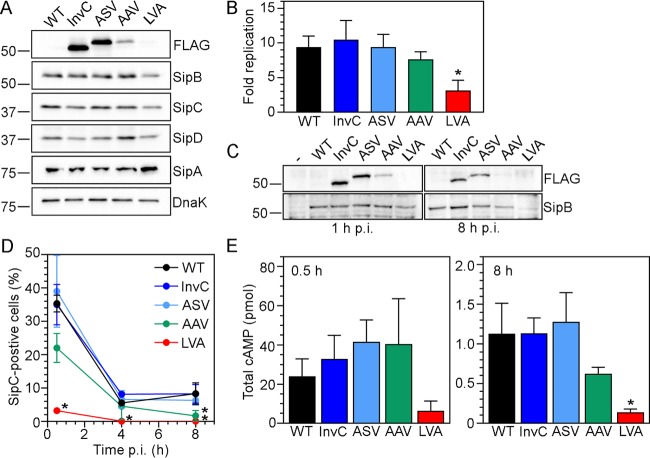
FIG 2 .
Altered InvC stability leads to tunable intracellular T3SS1 activity. (A) Immunoblotting detection of T3SS1 components in broth-grown bacteria. S. Typhimurium ΔinvC tetRA-invC-FLAG (InvC), ΔinvC tetRA-invC-FLAG(ASV), ΔinvC tetRA-invC-FLAG(AAV), and ΔinvC tetRA-invC-FLAG(LVA) subcultures were grown in the presence of 100 ng/ml ATc for 1.5 h to induce InvC-FLAG synthesis. Lysates from these strains and WT bacteria were probed with anti-FLAG (to detect InvC-FLAG), anti-SipB (T3SS1 translocator protein), anti-SipC (T3SS1 translocator protein), anti-SipD (T3SS1 tip complex protein), anti-SipA (T3SS1 effector), and anti-DnaK (loading control) antibodies. Blots are representative of two independent experiments. (B) Intracellular replication. Subcultures were grown as described for panel A and used to infect HeLa epithelial cells. MOIs were adjusted so that equivalent bacterial numbers were internalized for all strains. Monolayers were lysed for bacterial enumeration at 1 h and 8 h p.i. The fold replication over this timeframe is shown (mean ± SD; n ≥ 7 experiments). An asterisk denotes significantly different results from WT bacteria. (C) Immunoblotting detection of T3SS1 components during infection. HeLa cells were infected as described for panel B, and monolayers were collected at 1 h and 8 h p.i. and subjected to immunoblotting with anti-FLAG and anti-SipB antibodies. Samples were standardized to an equal number of bacteria for each time point. Blots are representative of three independent experiments. (D) Time course of SipC delivery into epithelial cells. HeLa cells were infected with S. Typhimurium WT and ATc-induced bacterial subcultures (harboring pFPV-mCherry) as described for panel B. Monolayers were fixed at 0.5 h, 4 h, and 8 h p.i. and immunostained for SipC. The percentage of infected cells positive for SipC signal was scored by fluorescence microscopy. Asterisks indicate data significantly different from WT. (E) SopB-CyaA translocation. HeLa epithelial cells were infected as described for panel B with strains harboring a SopB-CyaA plasmid. Lysates were collected at 0.5 h and 8 h p.i. and subjected to an ELISA for cAMP quantification (total cAMP/well). For reference, cAMP levels in WT bacteria-infected lysates (i.e., no SopB-CyaA plasmid) were 0.24 ± 0.15 pmol/well and 0.10 ± 0.044 pmol/well at 0.5 h and 8 h p.i., respectively. An asterisk denotes a result significantly different from that of WT SopB-CyaA bacteria.