Abstract
Abstract objective
To evaluate the validity of the Medication Adherence Self-Report Inventory (MASRI) questionnaire in determining antimuscarinic drugs adherence in patients with urinary incontinence (UI).
Patients and methods
In all, 629 patients [355 (56.4%) women and 274 (43.6%) men], aged 18–65 years, were included. All patients were prescribed antimuscarinic drugs and treatment adherence was tested at the start, and after 4, 8 and 12 weeks using the MASRI. The standard of external monitoring was the Brief Medication Questionnaire (BMQ) and visual count of the remaining pills. The functional status of the lower urinary tract was tested using voiding diaries and uroflowmetry.
Results
The correlation between indicators of adherence according to the MASRI and screen mode of the BMQ was r = 0.84 (P ≤ 0.01), r = 0.72 (P ≤ 0.01), r = 0.7 (P ≤ 0.05) at 4, 8 and 12 weeks of follow-up, respectively, which indicated a satisfactory competitive validity. In the study of the discriminant validity, we found that non-adherent patients were correctly identified according to the MASRI in 96.2%, 96.9% and 96.2% of cases at 4, 8 and 12 weeks of follow-up, respectively. The values of the positive likelihood ratio (7.92, 10.81, and 12.8 at 4, 8 and 12 weeks of follow-up, respectively) were quite acceptable for the adherence forecast. The receiver operating characteristic analysis revealed a failure of the null hypothesis of the excess/insufficient discrimination power of the MASRI. The correlation between the percentage of non-adherent patients and the percentage of patients with impaired lower urinary tract function according to uroflowmetry data was r = 0.55 (P ≤ 0.05) at 4 weeks; r = 0.59 (P ≤ 0.05) at 8 weeks; and r = 0.62 (P ≤ 0.01) at 12 weeks.
Conclusion
The MASRI questionnaire is highly constructive, competitive, has discriminant validity, and is suitable for self-assessment of treatment adherence in patients with UI taking antimuscarinics. Using the MASRI is less costly and faster compared with other assessment tools.
Abbreviations: AUC, area under the curve; BMQ, Brief Medication Questionnaire; ICIQ-SF, International Consultation on Incontinence Questionnaire-Short Form; LUT, lower urinary tract; MASRI, Medication Adherence Self-Report Inventory; OAB, overactive bladder; ROC, Receiver operating characteristic; (M)(S)(U)UI, (mixed) (stress) (urgency) urinary incontinence
Keywords: Self-evaluation, Questionnaire, Antimuscarinic, Adherence, Urinary incontinence
Introduction
Urinary incontinence (UI) is a common complaint, which the ICS defines as ‘any involuntary leakage of urine’ and is classified into urgency UI (UUI), stress UI (SUI), and mixed UI (MUI) [1]. The prevalence of UI is usually 29% [2] to 41.4–44% [3], [4], but can be as high as 54.8% [5], [6]. Direct economic costs of UI treatment are very high and comparable to the cost of treatment of diabetes and chronic obstructive pulmonary disease. UI affects productivity and is accompanied with frequent daily work breaks [7], [8], and it negatively affects health-related quality of life [9]. Today, there are numerous effective and safe drugs for the treatment of UI (the first-line being antimuscarinics), but the results of their use often differ from the expected [10], [11], [12], [13]. The important factor affecting the efficacy of UI treatment is patient’s adherence to the prescribed drugs. Inaccurate and incomplete adherence to the requirements of a physician can lead to drug replacement, increasing doses being prescribed, and, eventually, to a reduction in the treatment efficacy and an increase in its cost [14], [15].
The adherence level, according to practitioners often appears to be lower than that reported in randomised clinical studies [16]. Tools used for studying the level of adherence to treatment include, as a rule, electronic devices recording the number of pills administered, pharmacy records, pill count, and some interviewer questionnaires. Electronic pill counters are often used in randomised clinical studies, but are an expensive and inconvenient tool in clinical practice [17], [18]. The interviewer questionnaires currently used in clinical practice for measuring adherence and determining reasons for the refusal of treatment, e.g. the Brief Medication Questionnaire (BMQ), have high construct validity as compared with other tools. However, these questionnaires may also be too complicated and time-consuming for patients [19], [20].
Today, to measure treatment adherence and evaluate difficulties in following physician’s instructions, the Medication Adherence Self-Report Inventory (MASRI) is proposed for use. This brief tool for evaluating adherence and reasons for the refusal of treatment has proved itself to be effective in studies in patients with various chronic diseases, as well as in women with overactive bladder (OAB) [15], [21]. However, to date, no one has studied its validity for evaluating adherence and possible reasons for non-adherence to prescribing instructions in patients with various forms of UI in the population in general. Also, the correlation of objective indicators of the functional state of the lower urinary tract (LUT) with MASRI data on treatment adherence remains unstudied, which could provide additional information on the validity of this questionnaire.
The objective of the present study was to evaluate the MASRI efficacy compared to the standard BMQ questionnaire in individuals of both sexes with UI, with objective control of the LUT state.
Patients and methods
This prospective randomised study was conducted in the Urology Department of the City Polyclinic No. 3 from 9 September to 31 December 2015. It involved 629 patients [355 (56.4%) women and 274 (43.6%) men]. The criteria for inclusion were: age 18–65 years, ≥1 UI episode/day during the month preceding the study. The group included patients who were identified to have UUI [International Classification of Diseases, 10th Revision, Clinical Modification (ICD-10-CM) N39.41] and MUI (ICD-10-CM N39.46). Voiding diaries were used for evaluating the number of UI episodes [22]. The exclusion criteria were: SUI, contraindication to antimuscarinics and their current or recent (<6 months before) use, severe neurological diseases, current or chronic UTIs, acute urinary retention, a reduction of the QT interval, and terminal cancer. The study protocol was approved by the Ethics Commission of the Far Eastern Federal University. All patients signed a document of informed consent for participating in the study. The principles of the Declaration of Helsinki were used in planning and implementing the study.
At the preliminary stage of the study, all patients completed voiding diaries over a 1-month period. To exclude symptoms of OAB they also completed the Overactive Bladder Questionnaire-Short Form (OABq-SF). Further, for those who did not have OAB symptoms but had only UI complaints, uroflowmetry and completion of the International Consultation on Incontinence Questionnaire-Short Form (ICIQ-SF) were used to clarify the diagnosis. ICIQ-SF includes two main sections; subsections three, four and five in it describe LUTS, including the number of UI episodes. Symptoms severity is assessed using a scale from zero to 21 points. All patients were instructed to take the antimuscarinics prescribed by their urologist and these included: oxybutynin 5 mg twice a day, tolterodine 2 mg twice a day, trospium 5 mg twice a day, solifenacin 5 mg once a day, darifenacin 7.5 mg once a day. The schedule of visits included a primary visit and three subsequent visits at 4, 8 and 12 weeks after treatment initiation. All patients were instructed of the need to have the rest of their antimuscarinics with them during all subsequent visits. During the second visit (4 weeks after the active phase of the study began) in patients where an effect of treatment was not noted, either the antimuscarinic dose was increased (doubled) or they changed to another antimuscarinic. One of objectives of the visit was to evaluate the patients’ need for changing a drug or increasing its dosage. Treatment adherence was evaluated at 4, 8 and 12 weeks of follow-up using the BMQ, MASRI, and visual count of the remaining pills. The functional state of the LUT was evaluated using uroflowmetry and voiding diaries.
The MASRI is a questionnaire for self-evaluation of treatment adherence, and consists of a visual analogue scale, which helps to measure the adherence level in the range from zero to 100%. The MASRI also includes 12 questions divided into two scales used to help patients to identify the adherence level more confidently, but they are not used for evaluation themselves. The MASRI has been used to evaluate adherence in several prospective studies [23], as well as to evaluate adherence to fesoterodine treatment in women with OAB [15]. The BMQ is a tool for evaluating treatment adherence and reasons for refusal of it, using an interviewing method. We used the BMQ as an external standard for the study. The BMQ contains three scales reflecting the level of adherence to the regimen, as well as the level of belief and recall. In addition, patient’s answers are used to calculate the number of missed doses. Previously, it was shown that the BMQ has a good specificity, positive predictive value, and overall accuracy [19], [24], [28], [25]. The functional state of the LUT was evaluated using uroflowmetry (during patients’ visits) [26] and voiding diaries (throughout the study) [27], and any side-effects were recorded.
In summing and analysing all continuous variables the median and interquartile range were used. Describing categorical variables was performed using absolute and relative sign incidence.
The design validity, an extent of analysing the evaluation design tool, was evaluated by determining a relationship between non-adherence according to the MASRI and the presence of a belief barrier on the BMQ confidence monitor. We assumed that patients, who according to the MASRI score were non-adherent, were much more likely to have of a belief barrier than patients who were adherent to the drug. We compared the ratio of patients reporting a belief barrier, and patients who were adherent/non-adherent to treatment using the chi-squared test.
On the basis of previous similar studies, we assumed that the MASRI was an adequate tool, easily implemented in clinical practice for evaluating adherence to and reasons for the refusal of treatment. Constructive validity of the new tool was examined by comparing values of the MASRI ‘adherence’ scale and the BMQ ‘belief’ scale. A working hypothesis involved the assumption that patients with UI, who refused treatment according to the MASRI score, are much more likely to have low confidence in a good treatment outcome according to the BMQ score (the chi-square test was used to compare two samples of patients).
Criterion-competitive validity of the new tool was examined by comparing the number of refusals according to the MASRI and the number of missed doses on the BMQ adherence scale, as well as the results of the visual pill count. The calculation of correlation was performed using Spearman’s correlation coefficient.
The ability of the MASRI to identify a difference between quantitative indices of signs in different groups (discriminant validity) was determined by the tool efficacy when comparing patients adherent and non-adherent to treatment. The BMQ data and the visual pill count were used as an external standard. The significance of differences was determined using the chi-squared test.
Correlation of the tool with the objective state of the LUT was analysed by comparing variability curves for the percentage of patients who were adherent to treatment with curves reflecting average values of objective data of uroflowmetry and voiding diaries. The significance of differences was evaluated by the chi-squared test, and correlation using the Spearman’s correlation coefficient.
Receiver operating characteristic (ROC) analysis was used to evaluate the optimum ‘threshold’ of data that could unreliably indicate treatment adherence (comparison with BMQ results). Also, we determined the specificity, sensitivity, and likelihood ratios for the MASRI to evaluate the probability of the refusal of treatment with the calculation of 95% CIs for each ratio. Differences were considered significant with a P < 0.05 and all P values were two-sided.
All statistical analyses were performed using the Statistical Analysis System (SAS) version 8.0.2 (SAS Institute Inc., Cary, NC, USA).
Results
In all, 89 (14.1%) patients [57 men (9.1%) and 32 women (5.1%)] of the initial 629 patients ceased to participate in the study for various reasons. We also failed to collect complete MASRI data in nine (1.3%) patients. Thus, the adherence data of 531 (84.4%) patients were available for analysis after 12 weeks of follow-up (Table 1). Patient groups at the start and finish of the study had statistically homogeneous demographics and baseline characteristics.
Table1.
Baseline sociodemographic and medical characteristics of the patients.
| Characteristic | Start | Finish |
|---|---|---|
| Number of patients | 629 | 531 |
| Age, year, mean (SD) | 51.7 (12.4) | 53.9 (8.4) |
| N (%) | ||
| The level of education | ||
| Higher (university) | 265 (42.1) | 251 (49.4) |
| Secondary general | 47 (7.5) | 61 (12.0) |
| Secondary professional | 218 (34.6) | 196 (38.5) |
| Female | 355 (56.4) | 320 (62.9) |
| Married | 429 (68.2) | 374 (73.6) |
| Lives in the city district | 455 (72.3) | 389 (76.6) |
| Prior anticholinergic use | 518 (82.3) | 438 (86.2) |
| Prior non-medical therapy for OAB | 67 (10.6) | 61 (12.0) |
| Mean (SD) | ||
| Number of other medications | 3.9 (1.2) | 4.1 (1.3) |
| Incontinence Impact Questionnaire score | 26.7 (9.2) | 24.9 (12.8) |
| Voiding diary | ||
| Number episodes of urination | 11.8 (3.2) | 7.8 (2.3) |
| Number episodes of UI | 4.6 (1.3) | 1.7 (1.1) |
| Uroflowmetry | ||
| Volume of urinary bladder, mL | 190.4 (36.9) | 293.7 (31.8) |
| Qaver, mL/s | 19.7 (4.8) | 17.2 (5.9) |
Note. Qaver the average rate of urination.
Analysis of the BMQ results (regimen screen) revealed that, after 4 weeks of the study low treatment adherence was typical for 131 patients (20.8%), after 8 weeks for 195 (31.0%), and after 12 weeks for 267 (42.4%) patients.
In the study of construct validity (Table 2), we found that amongst patients who were non-adherent according to the MASRI data, 74% (4 weeks), 81.5% (8 weeks) and 83.5% (12 weeks) of the patients had a belief barrier according to the BMQ data. The difference between the percentage of adherent and non-adherent patients according to the MASRI data was significant throughout the experiment (P ≤ 0.01 at 4 weeks; P ≤ 0.01 at 8 weeks; P ≤ 0.05 at 12 weeks). The correlation between the change in the percentage of non-adherent patients according to MASRI and the percentage of patients having a belief barrier according to the BMQ was r = 0.85 (P ≤ 0.01), r = 0.89 (P ≤ 0.01), and r = 0.89 (P ≤ 0.05) at 4, 8, and 12 weeks, respectively.
Table 2.
Belief barriers and rate of medication omission (screens of BMQ) for patients with <80% and ≥80% adherence to treatment (MASRI).
| Follow-up |
||||
|---|---|---|---|---|
| Level of adherence | 4 weeks, n (%) (n = 593) |
8 weeks, n (%) (n = 549) |
12 weeks, n (%) (n = 531) |
Screens BMQ |
| MASRI < 80% 1 | 97/131 (74.0) | 159/195 (81.5) | 223/267 (83.5) | Belief barriers |
| MASRI ≥ 80% 2 | 46/462 (9.0)** | 79/354 (22.3)** | 95/264 (36.0)* | |
| MASRI < 80% 3 | 112/131(85.5) | 154/195 (79.0) | 213/267 (79.8) | Regimen |
| MASRI ≥ 80% 4 | 76/462 (16.4)** | 52/354 (14.7)** | 34/264 (12.7)* | |
P ≤ 0.05.
P ≤ 0.01 for comparison between patients with <80% and ≥80% MASRI.
There was a high level of correlation (r = 0.84, P ≤ 0.01; r = 0 72, P ≤ 0.01; r = 0.7, P ≤ 0.05 at 4, 8 and 12 weeks of follow-up, respectively) between the change in percentage of patients with missed doses in the BMQ regimen screen and the percentage of patients non-adherent according to the MASRI. The results of the visual pill count were also closely associated with the results of adherence evaluation using the MASRI (r = 0.65–81, P ≤ 0.05). These data support the hypothesis of a high competitive validity of the instrument.
In the study of the discriminant validity, we determined the confidence level of the adherence detection compared to the BMQ data. According to the MASRI, patients had a low adherence level at 4 weeks, at 126 (21.2%) vs 131 (22.1%) patients according to the BMQ data. In all, 189 (34.4%) patients showed a low adherence at 8 weeks according to MASRI vs 195 (35.5%) according to the BMQ data. In all, 257 (48.4%) patients had low adherence according to MASRI at 12 weeks vs 267 (50.3%) according to the BMQ data.
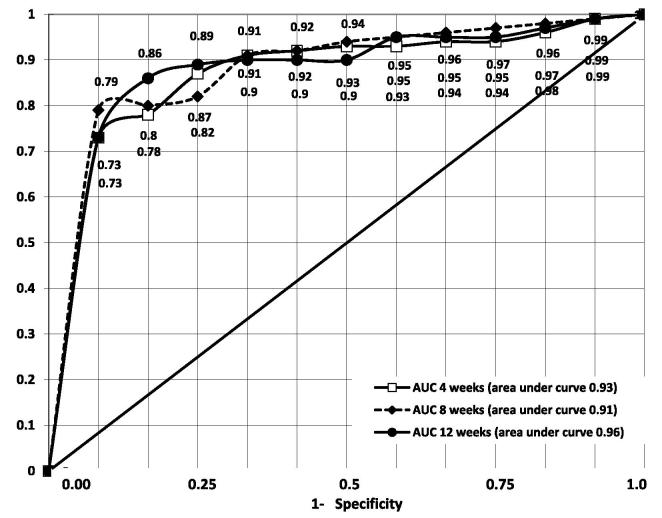
At 8 and 12 weeks of the study, the values of indicators of LUT function were significantly different in groups with different levels of treatment adherence according to the MASRI (Table 3). The correlation between the percentage of patients non-adherent to antimuscarinics according to the MASRI and the percentage of patients with impaired function of the LUT according to the voiding diaries was r = 0.45 (P ≤ 0.05) at 4 weeks; r = 0.51 (P ≤ 0.05) at 8 weeks, and r = 0.59 (P ≤ 0.01) at 12 weeks of follow-up. The correlation between the percentage of non-adherent patients and the percentage of patients with impaired function of the LUT according to uroflowmetry was: r = 0.55 (P ≤ 0.05) at 4 weeks; r = 0.59 (P ≤ 0.05) at 8 weeks; and r = 0.62 (P ≤ 0.01) at 12 weeks of follow-up. When comparing the data for men and women, it was established that the status of urodynamic markers was significantly different at all control points of observation (start, 4, 8, 12 weeks; P ≤ 0.05 in all cases), but the direction of urodynamics changes amongst those committed and not committed to treatment was not dependent on sex. In the ROC analysis, the area under the curve (AUC) reflected the acceptable values of the variables tending to 1 (Fig. 1). The findings rejected the null hypothesis of the AUC validity at values close to 0.5. The AUC was 0.93 ± 0.03 after 4 weeks from the start of the study, 0.91 ± 0.05 after 8 weeks, and 0.96 ± 0.03 after 12 weeks. The 95% CIs were set up for the calculations. Thus, the null hypothesis of the discrimination power was rejected for all time intervals.
Table 3.
Changes in the function of the LUT in men and women with different levels of commitment to the prescribed antimuscarinic regimen assessed by the MASRI (n = 629; men = 274, women = 355).
| Variable | Adherence on MASRI at follow-up |
||||||
|---|---|---|---|---|---|---|---|
| Start | 4 weeks |
8 weeks |
12 weeks |
||||
| <80% | ≥80% | <80% | ≥80% | <80% | ≥80% | ||
| Voiding diaries, number of episodes/day, mean (SD) | |||||||
| Frequency of urination | |||||||
| Men | 8.5 (2.2) | 8.1 (1.5) | 7.3 (2.7) | 8.0 (1.1) | 6.5 (1.3) | 8.2 (1.4) | 5.9 (1.2)* |
| Women | 11.3 (1.8) | 11.0 (1.6) | 8.3 (1.4) | 10.5 (2.6) | 7.2 (1.5) | 9.8 (1.5) | 6.3 (1.7)* |
| Urgency | |||||||
| Men | 4.3 (1.7) | 4.0 (1.1) | 2.9 (1.8) | 3.4 (1.6) | 2.1 (0.9) | 3.4 (1.0) | 1.1 (0.8)* |
| Women | 7.5 (1.3) | 7.2 (1.8) | 3.2 (1.6) | 6.9 (1.3) | 2.6 (1.4)* | 5.9 (1.2) | 2.1 (1.1)* |
| UUI | |||||||
| Men | 2.3 (0.4) | 4.6 (1.6) | 2.5 (1.0) | 4.2 (1.1) | 1.9 (0.7)* | 3.4 (0.9) | 1.7 (0.6)* |
| Women | 5.1 (1.6) | 4.9 (1.1) | 3.2 (0.7) | 4.5 (0.5) | 2.2 (0.8)* | 3.8 (0/9) | 1.9 (0.7)* |
| Uroflowmetry, mean (SD) | |||||||
| Qaver, mL/s | |||||||
| Men | 13.4 (4.7) | 14.0 (8.1) | 17.2 (6.1) | 14.3 (3.9) | 17.6 (4.8) | 14.9 (4.8) | 17.9 (3.5) |
| Women | 16.9 (9.0) | 15.7 (5.8) | 18.5 (4.2) | 16.6 (6.5) | 18.9 (6.9) | 16.9 (9.5) | 19.5 (4.8) |
| Bladder volume, mL | |||||||
| Men | 233.5 | 241.6 | 299.5 | 238.3 | 341.6 | 255.7 | 356.7 |
| Women | 198.5 | 211.7 | 267.8 | 246.7 | 302.7 | 247.8 | 317.0 |
Qaver, average flow rate.
P < 0.05 for comparison between patients with <80% and ≥80% MASRI.
Fig. 1.
ROC curve of the MASRI as compared to the BMQ at 4, 8 and 12 weeks of follow-up (n = 629).
The most acceptable ratio of sensitivity and specificity levels was recorded using a 90% threshold as a conventional barrier of adherence to antimuscarinic treatment (Table 4). Sensitivity and specificity of the method at this level were 89% and 93% at 4 weeks, 85% and 91% at 8 weeks, and 89% and 91% at 12 weeks of follow-up. When using the 80% threshold of the adherence barrier according to the MASRI the high sensitivity of the method, but its relatively low specificity was noted.
Table 4.
Significance of different thresholds on the MASRI as compared to the BMQ at 4, 8 and 12 weeks study adherence to the antimuscarinic regimen (n = 629).
| MASRI threshold | Sensitivity | Specificity | Positive likelihood ratio | |
|---|---|---|---|---|
| 4 weeks | ≥80% | 0.93 (0.18) | 0.78 (0.19) | 7.92 (3.14) |
| ≥90% | 0.90 (0.12) | 0.94 (0.14) | 8.82 (1.95) | |
| ≥95% | 0.85 (0.07) | 0.95 (0.21) | 16.9 (4.98) | |
| 8 weeks | ≥80% | 0.89 (0.13) | 0.82 (0.14) | 7.27 (4.22) |
| ≥90% | 0.86 (0.15) | 0.92 (0.11) | 10.81 (2.93) | |
| ≥95% | 0.78 (0.09) | 0.95 (0.08) | 21.75 (6.18) | |
| 12 weeks | ≥80% | 0.95 (0.08) | 0.79 (0.07) | 8.84 (6.8) |
| ≥90% | 0.90 (0.20) | 0.92 (0.10) | 12.8 (3.7) | |
| ≥95% | 0.82 (0.14) | 0.93 (0.8) | 19.9 (4.6) | |
To explore the possibility of extrapolating the data to the population, we calculated the positive likelihood ratio. When using the 80% threshold of the adherence barrier, this figure was found to be 7.92, 7.27 and 8.84, respectively, at 4, 8 and 12 weeks of follow-up. These values are lower than at 90% adherence barrier, but nevertheless acceptable for the requirements performance forecast.
Discussion
In the present study, we found that there was a high level of correlation (r = 0.85, P ≤ 0.01) between the percentage of non-adherent patients according to the MASRI data and the percentage of patients who had a belief barrier according to the BMQ, indicating the construct validity of the instrument. The hypothesis of the significant level of the competition validity of this tool is supported by data on the close relationship of the percentage of patients with missed doses in the BMQ regimen screen and the percentage of patients who were non-adherent according to the MASRI. The correlation between the indices was r = 0.84 (P ≤ 0.01), r = 0.72 (P ≤ 0.01), and r = 0.7 (P ≤ 0.05), at 4, 8 and 12 weeks of follow-up, respectively. The results of visual antimuscarinic pill count were also closely associated with the results of the adherence evaluation using the MASRI. Non-adherent patients were correctly identified according to the MASRI in 96.2%, 96.9% and 96.2% of cases at 4, 8 and 12 weeks of follow-up, respectively (external standard the BMQ). These data are similar to the results obtained in the study of the discriminant validity of the MASRI for OAB in women [15] and a number of other chronic diseases [21].
The dynamics of the functional state of the LUT in treatment with antimuscarinics in some cases did not coincide with the findings of other researchers [10], [11], and was even slightly different from the results obtained in our previous studies [13]. However, the efficacy and safety of the specific antimuscarinic drugs with different pharmacodynamics characteristics have been studied during these experiments. The present study used different antimuscarinic drugs, leading to a mixed therapeutic effect. However, there was a correlation between the percentage of non-adherent patients and the percentage of patients with impaired LUT function according to uroflowmetry and voiding diary data.
During the ROC analysis it was found that the null hypothesis of excessive discrimination power of the tool was rejected at all time intervals. The curve analysis showed that the 90% threshold in the evaluation of adherence would be optimal for determining the resistance of patients, and the values of the positive likelihood ratio were quite acceptable for the adherence forecast. Considering the low adherence to doctor recommendations, which is typical for the patients taking antimuscarinics [10], [11], [29], [30], the use of the 80% adherence evaluation barrier may, in our opinion, be considered optimal when using the MASRI as a forecasting tool.
The level of medication non-adherence in our present study was slightly higher than in standard randomised clinical trials, but was consistent with clinical practice [16], [30], [31]. This may be due to the design of the study, aimed at maximum approximation of the ways of interaction with the patient in routine clinical practice. We consider that the important result is the support of our assumption that, by the evaluation of adherence, the MASRI may be an effective alternative to the electronic count of pills, which may be accompanied by mistakes and be extremely time-consuming and cumbersome [14]. The BMQ questionnaire has a higher sensitivity and specificity compared with other existing methods; however, it requires considerable survey time and the employment of a special interviewer, which may be associated with additional costs and inconveniences in clinical practice [32].
As it has been shown that the sensitivity, specificity and likelihood ratio of the MASRI at the 4-week follow-up are valid for an adequate assessment of adherence, we believe it would be advisable to use this tool in the initial phase of treatment with antimuscarinic drugs. In cases where patients are identified as non-adherent to treatment, it might be appropriate to use the BMQ for an in-depth study of the causes of failure to treat or patients’ doubts as to antimuscarinic drugs benefits.
The MASRI is a tool primarily intended for use in clinical practice. Thus, the results presented in our present study may be useful for practical use by doctors to assess the risk of refused treatment or adherence reduction in patients with UI. Of course, we do not consider this as an exhaustive the study and evaluation of the MASRI. The present study is not free from certain limitations. In particular, the sample of patients was disproportionate on the basis of gender, which could distort the results. We consider that further research of the MASRI would be suitable for the study of the effectiveness of this tool in the evaluation of clinical outcomes in patients with the UI taking antimuscarinic drugs for a long time, as well as combined therapy. It also requires further study of validity of the proportional sample of patients by gender.
Conclusion
Our present study has shown that the MASRI is a valid tool for assessing adherence to treatment in patients with UI taking antimuscarinic drugs. The use of the MASRI is appropriate in clinical practice to reduce the time for diagnostic procedures, simplify the assessment, and reduce costs.
Conflict of interest
The authors claim there are no conflicts of interest between them or with any outside organisations.
Voiding Dysfunction/Female Urology
Footnotes
Peer review under responsibility of Arab Association of Urology.
References
- 1.Abrams P., Andersson K.E., Birder L. Fourth International Consultation on Incontinence Recommendations of the International Scientific Committee: evaluation and treatment of urinary incontinence, pelvic organ prolapse, and fecal incontinence. Neurourol Urodyn. 2010;29:213–240. doi: 10.1002/nau.20870. [DOI] [PubMed] [Google Scholar]
- 2.Altaweel W., Alharbi M. Urinary incontinence. Prevalence, risk factors, and impact on health related quality of life in Saudi women. Neurourol Urodyn. 2012;31:642–645. doi: 10.1002/nau.22201. [DOI] [PubMed] [Google Scholar]
- 3.Al-Badr A., Brasha H., Al-Raddadi R., Noorwali F., Ross S. Prevalence of urinary incontinence among Saudi women. Int J Gynaecol Obstet. 2012;117:160–163. doi: 10.1016/j.ijgo.2011.12.014. [DOI] [PubMed] [Google Scholar]
- 4.Sensoy N., Dogan N., Ozek B., Karaaslan L. Urinary incontinence in women: prevalence rates, risk factors and impact on quality of life. Pak J Med Sci. 2013;29:818–822. doi: 10.12669/pjms.293.3404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.El-Azab A., Mohammed E., Sabra H. The prevalence and risk factors of urinary incontinence and its influence on the quality of life among Egyptian women. Neurourol Urodyn. 2007;26:783–788. doi: 10.1002/nau.20412. [DOI] [PubMed] [Google Scholar]
- 6.Coyne K.S., Wein A., Nicholson S., Kvasz M., Chen C.I., Milsom I. Economic burden of urgency urinary incontinence in the United States: a systematic review. J Manag Care Pharm. 2014;20:130–140. doi: 10.18553/jmcp.2014.20.2.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sexton C.C., Coyne K.S., Vats V., Koppz S., Irwin D.E., Wagner T.H. Impact of overactive bladder on work productivity in the United States: results from EpiLUTS. Am J Manag Care. 2009;15(Suppl.):S98–S107. [PubMed] [Google Scholar]
- 8.Qin L., Luo X., Zou K.H., Snedecor S.J. Economic impact of using fesoterodine for the treatment of overactive bladder with urge urinary incontinence in a vulnerable elderly population in the United States. J Med Econ. 2016;19:229–235. doi: 10.3111/13696998.2015.1111893. [DOI] [PubMed] [Google Scholar]
- 9.Coyne K.S., Payne C., Bhattacharyya S.K., Revicki D.A., Thompson C., Corey R. The impact of urinary urgency and frequency on health-related quality of life in overactive bladder: results from a national community survey. Value Health. 2004;7:455–463. doi: 10.1111/j.1524-4733.2004.74008.x. [DOI] [PubMed] [Google Scholar]
- 10.Felicilda-Reynaldo R.F. A review of anticholinergic medications for overactive bladder symptoms. Medsurg Nurs. 2013;22:119–123. [PubMed] [Google Scholar]
- 11.Erdem N., Chu F. Management of overactive bladder disease and urge urinary incontinence in the elderly patient. Am J Med. 2006;119:29–36. doi: 10.1016/j.amjmed.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 12.Kosilov K., Loparev S., Ivanovskaya M., Kosilova L. Randomized controlled trial of cyclic and continuous therapy with trospium and solifenacin combination for severe overactive bladder in elderly patients with regard to patient compliance. Ther Adv Urol. 2014;6:215–223. doi: 10.1177/1756287214544896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kosilov K., Loparev S., Ivanovskaya M., Kosilova L. Effectiveness of combined high-dosed trospium and solifenacin depending on severity of OAB symptoms in elderly men and women under cyclic therapy. Cent European J Urol. 2014;67:43–48. doi: 10.5173/ceju.2014.01.art9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Basra R.K., Wagg A., Chapple C., Cardozo L., Castro-Diaz D., Pons M.E. A review of adherence to drug therapy in patients with overactive bladder. BJU Int. 2008;102:774–779. doi: 10.1111/j.1464-410X.2008.07769.x. [DOI] [PubMed] [Google Scholar]
- 15.Andy U.U., Harvie H.S., Smith A.L., Propert K.J., Bogner H.R., Arya L.A. Validation of a self-administered instrument to measure adherence to anticholinergic drugs in women with overactive bladder. Neurourol Urodyn. 2015;34:424–428. doi: 10.1002/nau.22605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gopal M., Haynes K., Bellamy S.L., Arya L.A. Discontinuation rates of anticholinergic medications used for the treatment of lower urinary tract symptoms. Obstet Gynecol. 2008;112:1311–1318. doi: 10.1097/AOG.0b013e31818e8aa4. [DOI] [PubMed] [Google Scholar]
- 17.Farmer K.C. Methods for measuring and monitoring medication regimen adherence in clinical trials and clinical practice. Clin Ther. 1999;21:1074–1090. doi: 10.1016/S0149-2918(99)80026-5. [DOI] [PubMed] [Google Scholar]
- 18.Farmer K.C. Medication adherence in health care: are we utilizing what we have learned? Clin Ther. 2011;33:1081–1083. doi: 10.1016/j.clinthera.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 19.Svarstad B.L., Chewning B.A., Sleath B.L., Claesson C. The Brief Medication Questionnaire: a tool for screening patient adherence and barriers to adherence. Patient Educ Couns. 1999;37:113–124. doi: 10.1016/s0738-3991(98)00107-4. [DOI] [PubMed] [Google Scholar]
- 20.Morisky D.E., Green L.W., Levine D.M. Concurrent and predictive validity of a self-reported measure of medication adherence. Med Care. 1986;24:67–74. doi: 10.1097/00005650-198601000-00007. [DOI] [PubMed] [Google Scholar]
- 21.Koneru S., Shishov M., Ware A., Farhey Y., Mongey A.B., Graham T.B. Effectively measuring adherence to medications for systemic lupus erythematosus in a clinical setting. Arthritis Rheum. 2007;57:1000–1006. doi: 10.1002/art.22898. [DOI] [PubMed] [Google Scholar]
- 22.Chapple C.R., Drake M.J., Van Kerrebroeck P., Cardozo L., Drogendijk T., Klaver M. Total urgency and frequency score as a measure of urgency and frequency in overactive bladder and storage lower urinary tract symptoms. BJU Int. 2014;113:696–703. doi: 10.1111/bju.12555. [DOI] [PubMed] [Google Scholar]
- 23.Daleboudt G.M., Broadbent E., McQuen F., Kaptein A.A. Intentional and unintentional treatment nonadherence in patients with systemic lupus erythematosus. Arthritis Care Res. 2011;63:342–350. doi: 10.1002/acr.20411. [DOI] [PubMed] [Google Scholar]
- 24.Krass I., Taylor S.J., Smith C., Armour C.L. Impact on medication use and adherence of Australian pharmacists’ diabetes care services. J Am Pharm Assoc. 2003;2005(45):33–40. doi: 10.1331/1544345052843093. [DOI] [PubMed] [Google Scholar]
- 25.Rickles N.M., Svarstad B.L. Relationships between multiple self-reported nonadherence measures and pharmacy records. Res Social Adm Pharm. 2007;3:363–377. doi: 10.1016/j.sapharm.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 26.Singh G., Lucas M., Dolan L., Knight S., Ramage C., Hobson P., United Kingdom Continence Society Minimum standards for urodynamic practice in the UK. Neurourol Urodyn. 2010;29:1365–1372. doi: 10.1002/nau.20883. [DOI] [PubMed] [Google Scholar]
- 27.Amundsen C.L., Parsons M., Cardozo L., Vella M., Webster G.D., Coats A.C. Bladder diary volume per void measurements in detrusor overactivity. J Urol. 2006;176:2530–2534. doi: 10.1016/j.juro.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 28.Kosilov K.V., Loparev S.A., Ivanovskaya M.A., Kosilova L.V. Comparative effectiveness of combined high-dosed Trospium and Solifenacin for severe OAB symptoms in age-related aspect. Int J Urol Nurs. 2015;9:108–113. [Google Scholar]
- 29.Kosilov K.V., Loparev S.A., Ivanovskaya M.A., Kosilova L.V. Effectiveness of solifenacin and trospium for managing of severe symptoms of overactive bladder in patients with benign prostatic hyperplasia. Am J Mens Health. 2016;10:157–163. doi: 10.1177/1557988315595692. [DOI] [PubMed] [Google Scholar]
- 30.Sexton C.C., Notte S.M., Maroulis C., Dmochowski R.R., Cardozo L., Subramanian D. Persistence and adherence in the treatment of overactive bladder syndrome with anticholinergic therapy: a systematic review of the literature. Int J Clin Pract. 2011;65:567–585. doi: 10.1111/j.1742-1241.2010.02626.x. [DOI] [PubMed] [Google Scholar]
- 31.Tunis S.R., Stryer D.B., Clancy C.M. Practical clinical trials: increasing the value of clinical research for decision making in clinical and health policy. JAMA. 2003;290:1624–1632. doi: 10.1001/jama.290.12.1624. [DOI] [PubMed] [Google Scholar]
- 32.Lavsa S.M., Holzworth A., Ansani N.T. Selection of a validated scale for measuring medication adherence. J Am Pharm Assoc. 2011;51:90–94. doi: 10.1331/JAPhA.2011.09154. [DOI] [PubMed] [Google Scholar]