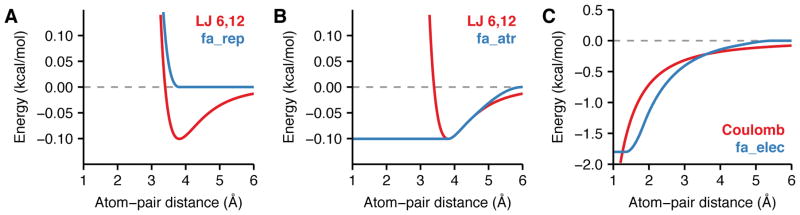
Figure 1. Van der Waals and electrostatics energies.
Comparison between pairwise energies of non-bonded atoms computed by Rosetta and the form computed by traditional molecular mechanics force fields. Here, the interaction between the backbone nitrogen and carbon are used as an example. (A) Lennard-Jones van der Waals energy with well-depths εNbb = 0.162 and εCbb = 0.063 and atomic radii rNbb = 1.763 and rCbb = 2.011 (red) and Rosetta fa_rep (blue). (B) Lennard-Jones van der Waals energy (red) and Rosetta fa_atr (blue). As the atom-pair distance approaches 6.0 Å, the fa_atr term smoothly approaches zero and deviates slightly from the original Lennard-Jones potential. (C) Coulomb electrostatics energy with a dielectric constant ε = 10, and partial charges pNbb = −0.604 and qCbb = 0.090 (red) compared with Rosetta fa_elec (blue). The fa_elec model is shifted to reach zero at the cutoff distance 6.0 Å.