Abstract
Background:
Vasovagal syncope (VVS) is the most common cause of syncope in children. Neuropeptide Y (NPY) plays an important role in the regulation of blood pressure (BP), as well as myocardial contractility. This study aimed to explore the role of plasma NPY in VVS in children.
Methods:
Fifty-six children who were diagnosed with VVS (VVS group) using head-up tilt test (HUT) and 31 healthy children who were selected as controls (control group) were enrolled. Plasma NPY concentrations were detected. The independent t-test was used to compare the data of the VVS group with those of the control group. The changes in plasma NPY levels in the VVS group during the HUT, as well as hemodynamic parameters, such as heart rate (HR), BP, total peripheral vascular resistance (TPVR), and cardiac output (CO), were evaluated using the paired t-test. Furthermore, the correlations between plasma NPY levels and hemodynamic parameters were analyzed using bivariate correlation analysis.
Results:
The BP, HR, and plasma NPY (0.34 ± 0.12 pg/ml vs. 0.46 ± 0.13 pg/ml) levels in the supine position were statistically low in the VVS group compared to levels in the control group (all P < 0.05). Plasma NPY levels were positively correlated with the HR (Pearson, R = 0.395, P < 0.001) and diastolic BP (Pearson, R = 0.311, P = 0.003) when patients were in the supine position. When patients in the VVS group were in the supine position, elevated TPVR (4.6 ± 3.7 mmHg·min−1·L−1 vs. 2.5 ± 1.0 mmHg·min−1·L−1, respectively, P < 0.001; 1 mmHg = 0.133 kPa) and reduced CO (1.0 ± 0.7 L/min vs. 2.4 ± 1.3 L/min, respectively, P < 0.001) were observed in the positive-response period compared with baseline values. The plasma NPY levels were positively correlated with TPVR (Spearman, R = 0.294, P = 0.028) but negatively correlated with CO in the positive-response period during HUT (Spearman, R = −0.318, P = 0.017).
Conclusions:
Plasma NPY may contribute to the pathogenesis of VVS by increasing the TPVR and decreasing the CO during orthostatic regulation.
Keywords: Cardiac Output, Children, Neuropeptide Y, Total Peripheral Vascular Resistance, Vasovagal Syncope
INTRODUCTION
Vasovagal syncope (VVS) is the most common cause of syncope in children, accounting for 60–70% of the causes of syncope.[1] Syncope or presyncope usually occurs during long-term standing or after a change from the supine or sitting position to the upright position, accompanied by a significant decline in blood pressure (BP) and/or bradycardia.[2] Recurrent episodes of syncope seriously influence children's physical and mental health, which leads to unavoidable anxiety in the parents, as well as the teachers. VVS is an important type of neurally mediated syncope, and its pathogenesis, which has not been fully understood, is thought to be associated with the imbalance between the sympathetic and the parasympathetic nervous systems, as well as the abnormal regulation of peripheral vascular tone.[3] Both autonomic nervous function and peripheral vascular tone can be regulated by a variety of humoral factors. Our previous studies revealed that children with neutrally mediated syncope exhibited an imbalance between vasoconstrictive factors (such as urotensin II)[4] and vasodilative factors (such as nitric oxide and hydrogen sulfide).[5,6] Neuropeptide Y (NPY), another biological active peptide, plays a crucial role in the regulation of BP, as well as myocardial contractility,[7] which are closely associated with the pathogenesis of VVS. However, the role of NPY in VVS in children has not been reported to date. To explain the pathogenesis of VVS, this study was designed to determine the plasma NPY levels in children with VVS and to explore the relationship between plasma NPY and hemodynamic parameters during the HUT.
METHODS
Ethical approval
This study was approved by the Ethics Committee of Peking University First Hospital. The guardians of all the participants were fully informed of the purpose and methods of the study and signed the informed consent form on behalf of the children who were enrolled in this study.
Participants
The VVS group consisted of 56 children who presented with complaints of syncope or presyncope from December 2013 to June 2015 at Peking University First Hospital. The diagnosis of VVS was made according to clinical features and the head-up tilt test (HUT). Children with cardiovascular, neurological, or metabolic diseases that could cause syncope or pseudosyncope were excluded. From March to June 2015, 31 healthy children were selected as the control group based on the history and clinical examination. All 31 children in the control group had no history of syncope or presyncope. All clinical data and blood samples were obtained from December 2013 to June 2015.
Diagnosis of vasovagal syncope
Diagnostic criteria
The diagnosis of VVS depends on the following criteria:[8] (1) usually, a school-aged child or adolescent; (2) a history of syncope or presyncope; (3) the presence of predisposing factors in most of the patients, such as long-term standing; (4) a positive response during the HUT; and (5) exclusion of other diseases that could cause syncope or a transient loss of consciousness.
Protocol for the head-up tilt test
The protocol for the HUT was according to the guidelines of diagnosis for children's syncope in China.[8] The subjects had fasted for at least 4 h. Medicines or food that could influence the activity of the autonomic nervous system were avoided for at least 5 half-lives before the test. The HUT was performed in a softly lit, quiet, and temperature-controlled room where the medical resuscitation facilities were available. In the beginning, children were kept supine on the tilt table for 10 min, and the baseline heart rate (HR), BP, and electrocardiograph were recorded. Next, the tilt table with a footboard that was electrically motorized positioned the patient, who was protected by a safety belt, to a tilt position at an angle of 60°. The electrocardiogram of the participants was continuously monitored by the Dash 2000 Multi-Lead Physiological Monitor (General Electric, NY, New York, USA) during the entire course of 45 min or until a positive response occurred.
Positive response during the head-up tilt test
Children with syncope or presyncope accompanied by any of the following responses during the HUT were considered to have a positive response:[8] (1) systolic BP <80 mmHg (1 mmHg = 0.133 kPa) or diastolic BP <50 mmHg or a decrease in the mean arterial pressure >25% from the baseline; (2) HR <75 beats per minute (bpm) for children aged 4–6 years; HR <65 bpm for children aged 7–8 years; and HR <60 bpm for children older than 8 years; (3) an electrocardiogram that showed sinus arrest or premature junctional contractions; and (4) atrioventricular block and cardiac arrest >3 s. The responses were classified as vasoinhibitory, cardioinhibitory, or mixed inhibitory. The vasoinhibitory type was characterized by a significant BP decrease without significant HR reduction. The cardioinhibitory type was characterized by a notable HR decrease without an obvious decrease in systolic pressure, and the mixed inhibitory type was characterized by both HR and BP decreases.
Other hemodynamic parameters obtained by the Finapres Medical System during the head-up tilt test
During the HUT, the Finapres Medical System (FMS) (FinometerPRO, FMS Company, Netherlands) was used for the continuous monitoring and recording of hemodynamic parameters, such as HR, BP, total peripheral vascular resistance (TPVR), and cardiac output (CO). BP was obtained noninvasively and continuously in time by a finger-cuff band around the middle finger of the participants using the system and BeatScope software (FMS Company, Netherlands). The software was capable of generating an aortic pressure wave according to the finger arterial pulse waveform using a mathematical model. As a result, left ventricular stroke volume was computed by the finger cuff of the FMS and BeatScope software. Meanwhile, CO and TPVR were calculated by the system according to the values of stroke volume, HR, and BP.
Determination of plasma neuropeptide Y concentration
Plasma NPY concentrations were detected for all of the participants in the supine position. For the VVS group, another blood sample was collected in the positive-response period during the HUT. Blood samples were obtained from the participants’ cubital veins after they fasted for at least 8 h. The blood samples were stored in vacuum tubes that contained ethylenediaminetetraacetic acid and aprotinin. Within 2 h after the collection, the blood samples were immediately kept at 4°C and centrifuged at 400 ×g for 20 min to extract the plasma. The plasma was later frozen and stored at −20°C until further analysis. Plasma NPY concentrations were determined using the enzyme immunoassay (Phoenix Pharmaceuticals, Burlingame, California, USA).
Statistical analysis
Statistical analysis was performed using SPSS Statistics version 21.0 software (International Business Machines Corp., Armonk, New York, USA). Numeration data were counted as cases. Measurement data were first tested for normal distribution using Kolmogorov–Smirnov methods. Data with normal distribution were presented as the mean ± standard deviation (SD) using the independent t-test to compare the data of the VVS group with the control group. Data with abnormal distribution were presented as the medians (minimum, maximum), and comparisons were performed by nonparametric tests. Comparisons of compositions were performed by the Chi-square test. The paired t-test was used to compare hemodynamic parameters in the supine position with those in the positive-response period during HUT. Bivariate correlation analysis was performed to explore the relationship between the plasma NPY levels and hemodynamic parameters using Pearson's method for data with normal distribution and Spearman's method for data with abnormal distribution. P < 0.05 was considered statistically significant.
RESULTS
Baseline characteristics of the vasovagal syncope group and the control group
There were no significant differences in age, gender composition, height, and body weight between the VVS group and the control group. However, the supine HR and BP were lower in the VVS group than in the control group [Table 1].
Table 1.
Baseline characteristics of the vasovagal syncope group and control group
| Items | Control group | VVS group | Statistics | P |
|---|---|---|---|---|
| Number (female/male) | 31 (19/12) | 56 (23/33) | 3.267* | 0.071 |
| Age (years) | 12.0 ± 0.8 | 11.2 ± 2.2 | 1.941† | 0.056 |
| Height (cm) | 146.2 ± 10.0 | 148.6 ± 14.7 | -0.932† | 0.354 |
| Weight (kg) | 45.9 ± 12.1 | 40.8 ± 12.7 | 1.831† | 0.071 |
| SBP (mmHg) | 110.0 ± 12.2 | 104.2 ± 11.0 | 2.279† | 0.025 |
| DBP (mmHg) | 69.1 ± 9.0 | 60.5 ± 9.3 | 4.172† | <0.001 |
| HR (bpm) | 85.1 ± 7.5 | 77.6 ± 11.6 | 3.630† | <0.001 |
Data are shown as n or mean ± SD. Data of age, height, body weight, blood pressure, and heart rate were in a normal distribution; thus, the independent t-test was used to compare the data of the VVS group with the control group. 1 mmHg = 0.133 kPa. *χ2 values; †t values. VVS: Vasovagal syncope; SBP: Systolic blood pressure; DBP: Diastolic blood pressure; HR: Heart rate; bpm: Beats per minute; SD: Standard deviation.
Baseline plasma neuropeptide Y concentration
The plasma NPY concentration was significantly low in the VVS group compared with the control group (0.34 ± 0.12 vs. 0.46 ± 0.13 pg/ml, P < 0.05).
Correlations between neuropeptide Y levels and hemodynamic parameters in the supine position
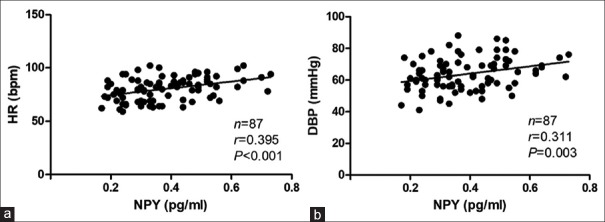
Plasma NPY levels in the supine position were positively correlated with the supine HR (Pearson, R = 0.395, P < 0.001) as well as the supine diastolic BP (Pearson, R = 0.311, P = 0.003) [Figure 1]. There was no significant correlation between the supine plasma NPY levels and the supine TPVR and CO levels in the VVS group.
Figure 1.
Correlations between baseline NPY levels and supine hemodynamic parameters. (a) Correlation between the plasma NPY levels and the supine HR. (b) Correlation between the plasma NPY levels and the supine diastolic BP. 1 mmHg = 0.133 kPa. NPY: Neuropeptide Y; HR: Heart rate; DBP: Diastolic blood pressure; bpm: Beats per minute.
Analysis of the change in plasma neuropeptide Y levels and hemodynamic parameters during the head-up tilt test in the vasovagal syncope group
In the VVS group, 34 patients showed a vasoinhibitory response, while the other 22 patients had a mixed inhibitory response during the HUT. No patients showed a cardioinhibitory response in this study. There was no significant difference in HR, BP, TPVR, or CO in the supine position between the two response types of VVS children. In the positive-response period, the BP and CO of the VVS patients sharply decreased, while the TPVR significantly increased. Furthermore, in children with a mixed inhibitory response, the BP and CO were even lower and the TPVR was much higher than that in children with a vasoinhibitory response. The HR obviously increased in children with a vasoinhibitory response and decreased in children with a mixed inhibitory response, which was consistent with the definition. The plasma NPY levels in the positive-response period during the HUT were not significantly different from those in the supine position [Table 2].
Table 2.
Changes in the hemodynamic parameters and NPY of the vasovagal syncope group during the HUT
| Items | Supine | HUT positive | Statistics | P |
|---|---|---|---|---|
| Number, n | 56 | 56 | ||
| VI type | 34 | 34 | – | – |
| MI type | 22 | 22 | – | – |
| SBP (mmHg) | 104.2 ± 11.0 | 60.8 ± 11.9 | 19.063* | <0.001 |
| VI type | 104.0 ± 10.7 | 63.8 ± 11.1 | 13.416* | <0.001 |
| MI type | 104.5 ± 11.5 | 56.1 ± 11.8 | 14.694* | <0.001 |
| DBP (mmHg) | 60.5 ± 9.3 | 41.4 ± 11.8 | 10.856* | <0.001 |
| VI type | 61.3 ± 10.0 | 46.3 ± 10.7 | 7.165* | <0.001 |
| MI type | 59.3 ± 8.5 | 34.0 ± 9.4 | 9.690* | <0.001 |
| HR (bpm) | ||||
| VI type | 77.4 ± 11.6 | 117.5 ± 24.0 | −8.957* | <0.001 |
| MI type | 77.9 ± 11.9 | 60.4 ± 20.7 | 3.695* | 0.001 |
| TPVR (mmHg·min−1·L−1) | 2.5 ± 1.0 | 4.6 ± 3.7 | −5.446* | <0.001 |
| VI type | 2.5 ± 1.0 | 3.8 ± 2.9 | −2.977* | 0.005 |
| MI type | 2.4 ± 1.0 | 6.0 ± 3.6 | −5.220* | <0.001 |
| CO (L/min) | 2.4 ± 1.3 | 1.0 ± 0.7 | 9.673* | <0.001 |
| VI type | 2.4 ± 1.1 | 1.1 ± 0.7 | 7.551* | <0.001 |
| MI type | 2.5 ± 1.4 | 0.7 ± 0.5 | 6.497* | <0.001 |
| NPY (pg/ml) | 0.34 ± 0.12 | 0.34 (0.08, 0.96) | −0.437† | 0.662 |
| VI type | 0.34 ± 0.12 | 0.34 (0.13, 0.96) | −0.282† | 0.778 |
| MI type | 0.33 ± 0.12 | 0.35 (0.08, 0.57) | −1.008† | 0.314 |
Data are shown as n, mean ± SD, or median (minimum, maximum). The paired t-test was used to compare hemodynamic parameters in the supine position with those in the positive-response period during the HUT. The independent t-test was used to compare the hemodynamic parameters between children in the two hemodynamic patterns. Nonparametric tests were used for the comparisons of NPY levels. 1 mmHg = 0.133 kPa. *t values; †Z values. –: Not applicable; VI: Vasoinhibitory; MI: Mixed inhibitory; HR: Heart rate; bpm: Beats per minute; TPVR: Total peripheral vascular resistance; CO: Cardiac output; NPY: Neuropeptide Y; SD: Standard deviation; HUT: Head-up tilt; SBP: Systolic blood pressure; DBP: Diastolic blood pressure.
Correlations between the upright plasma neuropeptide Y levels and hemodynamic parameters in the positive-response period during the head-up tilt test
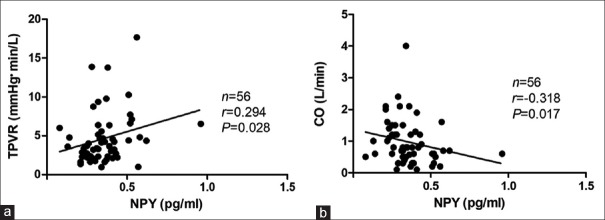
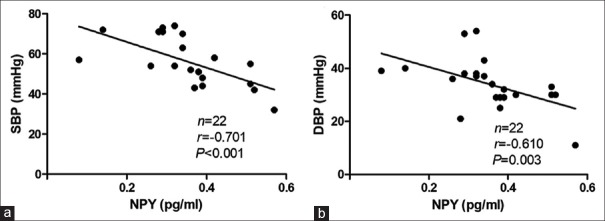
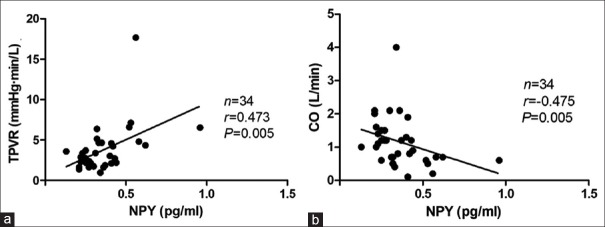
In the positive-response period during the HUT, the plasma NPY levels were positively correlated with the TPVR (Spearman, R = 0.294, P = 0.028) but negatively correlated with CO (Spearman, R = −0.318, P = 0.017) [Figure 2]. No statistical correlation was found between plasma NPY levels and HR or between plasma NPY levels and BP when the positive response occurred. Interestingly, when considering analysis of the mixed inhibitory type alone, the plasma NPY levels were not correlated with the TPVR or CO; however, they were negatively correlated with the BP (for systolic BP, R = −0.701, P < 0.001; for diastolic BP, R = −0.610, P = 0.003) when the positive response occurred [Figure 3]. Moreover, the plasma NPY levels in children with a vasoinhibitory response were positively correlated with the TPVR (Spearman, R = 0.473, P = 0.005) but negatively correlated with CO (Spearman, R = −0.475, P = 0.005), which was consistent with the entire VVS group [Figure 4].
Figure 2.
Correlations between upright NPY levels and hemodynamic parameters in the positive response period in vasovagal syncope children. (a) Correlation between the plasma NPY levels and the TPVR. (b) Correlation between the plasma NPY levels and the CO. 1 mmHg = 0.133 kPa. NPY: Neuropeptide Y; TPVR: Total peripheral vascular resistance; CO: Cardiac output.
Figure 3.
Correlations between upright NPY levels and hemodynamic parameters in the positive-response period in vasovagal syncope children with a mixed inhibitory response. (a) Correlation between the plasma NPY levels and the systolic blood pressure. (b) Correlation between the plasma NPY levels and the diastolic blood pressure. 1 mmHg = 0.133 kPa. NPY: Neuropeptide Y; SBP: Systolic blood pressure; DBP: Diastolic blood pressure.
Figure 4.
Correlations between upright NPY levels and hemodynamic parameters in the positive-response period in vasovagal syncope children with a vasoinhibitory response. (a) Correlation between the plasma NPY levels and the TPVR negatively. (b) Correlation between the plasma NPY levels and the CO. 1 mmHg = 0.133 kPa. NPY: Neuropeptide Y; TPVR: Total peripheral vascular resistance; CO: Cardiac output.
DISCUSSION
Neuropeptide Y distribution and its role in cardiovascular regulation
NPY was first isolated from the pig brain by Tatemoto et al. in 1982.[9] NPY is generated by neurons and widely distributed in the nervous system, as well as in the cardiovascular system, and is involved in extensive physiological activities when combined with its receptors.[10] In particular, the nerve fiber terminals that are rich in NPY are intensively distributed in the heart and blood vessels (especially in skeletal muscles), which are considered to take part in the regulation of HR and BP. In the peripheral nervous system, NPY participates in the regulation of autonomic nervous function by mediating vasoconstriction and increasing peripheral vascular resistance.[11] In addition, NPY can regulate the release of neurotransmitters by acting on the Y2 receptor on the presynaptic membrane of both sympathetic and parasympathetic nerve fibers, which may play a crucial role in HR regulation.[12] In the central nervous system, NPY is widely distributed in the cardiovascular regulation center. In research on rats, after injecting NPY into the lateral ventricle, a decrease in the peripheral sympathetic nervous activity and reduced norepinephrine release were observed, which resulted in decreases in both HR and BP.[13] Therefore, NPY in the central nervous system may have different regulatory effects on HR and BP compared to the NPY in the peripheral system.
Association between plasma neuropeptide Y and hemodynamics in vasovagal syncope children
Our study revealed that the plasma NPY levels in children with VVS were significantly lower compared to controls. In addition, the baseline HR and BP were lower in children with VVS compared to the control group. Positive correlations were observed between plasma NPY levels and HR, as well as diastolic BP, which indicates that the lower supine HR and BP in VVS children may be associated with the lower plasma NPY levels. Previous studies suggested that children with VVS had an imbalance in autonomic nervous function,[14] to be more specific, a decrease in the sympathetic tone and an increase in the vagal tone.[15] This finding may be one of the reasons for the slower baseline HR. Basic research showed that NPY could antagonize vagal bradycardia by stimulating presynaptic Y2 receptors on cardiac vagal nerve terminals and inhibit the release of the neurotransmitter acetylcholine.[12] Therefore, it can be speculated that the low plasma NPY levels in children with VVS may not antagonize the effect of the high vagal tone on HR, which leads to a relatively slow HR. The effects of plasma NPY on BP regulation seems to be more intelligible. It is believed that NPY mediates constriction of blood vessels and an increase in vascular resistance mainly via two mechanisms. First, plasma NPY can directly bind to the Y1 receptor in vascular smooth muscle cells to play a role in vasoconstriction, especially in the blood vessels of limb skeletal muscles.[15] Second, NPY, which coexists with norepinephrine in the sympathetic nerve terminals, can strengthen the vasoconstrictor effect of norepinephrine by acting on Y1 receptors in vascular endothelial cells.[16] The vasoconstrictor effects of angiotensin II and endothelin-1 can be significantly enhanced by NPY as well.[17] Therefore, the reduction in plasma NPY levels in children with VVS may be one of the reasons for the low supine BP.
In this study, children in the VVS group were classified as a vasoinhibitory type or a mixed type according to their hemodynamic response during the HUT. In the positive-response period of the HUT, the HR obviously increased in children with a vasoinhibitory response and decreased significantly in children with a mixed inhibitory response. Both types of children had a notable decrease in BP accompanied by an increase in TPVR and a reduction in CO. The classical pathogenesis of VVS was explained by the Bezold-Jarish reflex.[18] During the reflex, the sympathetic nervous system is first stimulated, which results in an increase in HR and myocardial constriction as a compensation to the reduction of venous return to the heart according to different triggers, for example, long-term standing. That finding is why an increase in HR can be observed in certain children during the HUT before the positive response appears. Next, the inhibitory reflex may be induced by the stimulation of mechanoreceptors in the heart, promoting parasympathetic activity that leads to bradycardia, vasodilation, and hypotension. As a result, it is not difficult to explain the reduction in CO by the decrease in BP and/or HR during the HUT. In addition, the significant increase in the TPVR may be a compensatory change.[19] Children with the vasoinhibitory type of VVS did not show a decrease in HR and had minor changes in BP, CO, and TPVR compared with children with the mixed inhibitory type of VVS. We speculated that children with the mixed inhibitory type may have increased excitability of the vagus nerve, and/or their cardiovascular system may be more sensitive to the vagus nerve. However, the reasons for the different responses during the HUT are unknown.
The plasma NPY levels in the positive-response period were positively correlated with the TPVR, which suggests that plasma NPY and peripheral vascular resistance increased in a parallel manner. This result was consistent with the results of previous studies.[15,16] The significant increase in the TPVR in the positive-response period during the HUT is believed to be a compensatory response to the decline in BP, and it could be proposed that plasma NPY may play a role in this compensatory mechanism. In addition, the plasma NPY levels in the positive-response period were negatively correlated with CO. Previous studies suggested that NPY can suppress myocardial contractility by inhibiting the energy metabolism of mitochondria in the cardiomyocytes.[20,21] Therefore, it is reasonable to propose that the upright plasma NPY may take part in the decline of CO in the positive-response period during the HUT by suppressing myocardial contractility. As mentioned above, the increase in the TPVR and the decline in CO are characteristic responses in children with VVS during the HUT. In summary, plasma NPY may play a role in the pathogenesis of VVS by increasing the TPVR and decreasing CO.
Limitations
There were several limitations in our study. NPY is widely distributed, but our research only involved NPY levels in the plasma, which did not represent the concentrations of NPY in the central nervous system or in the synapses. Thus, we revealed the role of NPY in VVS to a limited extent. More studies need to be designed to explore the possible comprehensive role of NPY in the pathogenesis of VVS.
To conclude, in this study, we first documented that the supine plasma NPY levels were significantly reduced in children with VVS. NPY might play a role in the pathogenesis of VVS by increasing the TPVR and decreasing CO during orthostatic regulation.
Financial support and sponsorship
This study was supported by a grant from the Beijing Municipal Science and Technology Major Project (No. Z171100001017253).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Qiang Shi
REFERENCES
- 1.Jin H, Du J. Pediatric syncope: Where are we now? Chin Med J. 2014;127:3681–3. doi: 10.3760/cma.j.issn.0366-6999.20132944. [PubMed] [Google Scholar]
- 2.Chen L, Yang YY, Wang C, Wang HW, Tian H, Zhang QY, et al. A multi-center study of hemodynamic characteristics exhibited by children with unexplained syncope. Chin Med J. 2006;119:2062–8. [PubMed] [Google Scholar]
- 3.Wieling W, Jardine DL, de Lange FJ, Brignole M, Nielsen HB, Stewart J, et al. Cardiac output and vasodilation in the vasovagal response: An analysis of the classic papers. Heart Rhythm. 2016;13:798–805. doi: 10.1016/j.hrthm.2015.11.023. doi: 10.1016/j.hrthm.2015.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liao Y, Du JB, Tang CS, Jin HF. Change and significance in the levels of plasma urotensin II and catestatin in children with postural orthostatic tachycardia syndrome (in Chinese) J Pek Univ (Health Sci) 2011;43:436–9. doi: 10.3969/j.issn.1671-167X.2011.03.024. [PubMed] [Google Scholar]
- 5.Yang J, Li H, Ochs T, Zhao J, Zhang Q, Du S, et al. Erythrocytic hydrogen sulfide production is increased in children with vasovagal syncope. J Pediatr. 2015;166:965–9. doi: 10.1016/j.jpeds.2014.12.021. doi: 10.1016/j.jpeds.2014.12.021. [DOI] [PubMed] [Google Scholar]
- 6.Liao Y, Chen S, Liu X, Zhang Q, Ai Y, Wang Y, et al. Flow-mediated vasodilation and endothelium function in children with postural orthostatic tachycardia syndrome. Am J Cardiol. 2010;106:378–82. doi: 10.1016/j.amjcard.2010.03.034. doi: 10.1016/j.amjcard.2010.03.034. [DOI] [PubMed] [Google Scholar]
- 7.Zhu P, Sun W, Zhang C, Song Z, Lin S. The role of neuropeptide Y in the pathophysiology of atherosclerotic cardiovascular disease. Int J Cardiol. 2016;220:235–41. doi: 10.1016/j.ijcard.2016.06.138. doi: 10.1016/j.ijcard.2016.06.138. [DOI] [PubMed] [Google Scholar]
- 8.Subspecialty Group of Cardiology, The Society of Pediatrics, Chinese Medical Association; Editorial Board, Chinese Journal of Pediatrics. Guidelines for diagnosis of syncope in children (in Chinese) Chin J Pediatr. 2009;47:99–101. doi: 10.3760/cma.j.issn.0578-1310.2009.02.006. [PubMed] [Google Scholar]
- 9.Tatemoto K, Carlquist M, Mutt V. Neuropeptide Y – A novel brain peptide with structural similarities to peptide YY and pancreatic polypeptide. Nature. 1982;296:659–60. doi: 10.1038/296659a0. [DOI] [PubMed] [Google Scholar]
- 10.Crnkovic S, Egemnazarov B, Jain P, Seay U, Gattinger N, Marsh LM, et al. NPY/Y1 receptor-mediated vasoconstrictory and proliferative effects in pulmonary hypertension. Br J Pharmacol. 2014;171:3895–907. doi: 10.1111/bph.12751. doi: 10.1111/bph.12751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hartl J, Dietrich P, Moleda L, Müller-Schilling M, Wiest R. Neuropeptide Y restores non-receptor-mediated vasoconstrictive action in superior mesenteric arteries in portal hypertension. Liver Int. 2015;35:2556–63. doi: 10.1111/liv.12874. doi: 10.1111/liv.12874. [DOI] [PubMed] [Google Scholar]
- 12.Ilebekk A, Björkman JA, Nordlander M. Influence of endogenous neuropeptide Y (NPY) on the sympathetic-parasympathetic interaction in the canine heart. J Cardiovasc Pharmacol. 2005;46:474–80. doi: 10.1097/01.fjc.0000177986.21929.d8. doi: 10.1097/01.fjc.0000177986.21929.d8. [DOI] [PubMed] [Google Scholar]
- 13.Morris MJ, Tortelli CF, Hart DP, Delbridge LM. Vascular and brain neuropeptide Y in banded and spontaneously hypertensive rats. Peptides. 2004;25:1313–9. doi: 10.1016/j.peptides.2004.05.006. doi: 10.1016/j.peptides.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 14.Zhao J, Du JB. Blood pressure variability in children with autonomous nerve mediated syncope (in Chinese) Chin J Pediatr. 2012;50:712–3. doi: 10.3760/cma.j.issn.0578-1310.2012.09.017. [PubMed] [Google Scholar]
- 15.Zheng HF, Wang C, Cao MJ, He ZX, Li MX, Lin P, et al. Analysis on heart rate variability in children with vasovagal syncope (in Chinese) J Clin Pediatr. 2006;24:361–4. doi: 10.3969/j.issn.1000-3606.2006.05.006. [Google Scholar]
- 16.Wier WG, Zang WJ, Lamont C, Raina H. Sympathetic neurogenic Ca2+ signalling in rat arteries: ATP, noradrenaline and neuropeptide Y. Exp Physiol. 2009;94:31–7. doi: 10.1113/expphysiol.2008.043638. doi: 10.1113/expphysiol.2008.043638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Linder L, Lautenschlager BM, Haefeli WE. Subconstrictor doses of neuropeptide Y potentiate alpha 1-adrenergic venoconstriction in vivo. Hypertension. 1996;28:483–7. doi: 10.1161/01.hyp.28.3.483. doi: 10.1161/01.HYP.28.3.483. [DOI] [PubMed] [Google Scholar]
- 18.Aviado DM, Guevara Aviado D. The Bezold-Jarisch reflex. A historical perspective of cardiopulmonary reflexes. Ann N Y Acad Sci. 2001;940:48–58. doi: 10.1111/j.1749-6632.2001.tb03666.x. [PubMed] [Google Scholar]
- 19.Stewart JM. Mechanisms of sympathetic regulation in orthostatic intolerance. J Appl Physiol. 2012;113:1659–68. doi: 10.1152/japplphysiol.00266.2012. doi: 10.1152/japplphysiol.00266.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luo G, Xu X, Guo W, Luo C, Wang H, Meng X, et al. Neuropeptide Y damages the integrity of mitochondrial structure and disrupts energy metabolism in cultured neonatal rat cardiomyocytes. Peptides. 2015;71:162–9. doi: 10.1016/j.peptides.2015.07.001. doi: 10.1016/j.peptides.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 21.Zverev AA, Anikina TA, Maslyukov PM, Zefirov TL. Role of neuropeptide Y in myocardial contractility of rats during early postnatal ontogeny. Bull Exp Biol Med. 2014;157:421–3. doi: 10.1007/s10517-014-2581-2. doi: 10.1007/s10517-014-2581-2. [DOI] [PubMed] [Google Scholar]