Abstract
Objectives
Efavirenz is currently suggested as an alternative to recommended antiretroviral (ARV) regimens by the Department of Health and Human Services for the treatment of HIV-1 in ARV-naive patients. A mid-dosing interval therapeutic range between 1,000 and 4,000 ng/mL for efavirenz has been proposed in the literature, with patients more likely to experience virologic failure below this range and adverse effects above. The current study reports an analysis of virologic outcome between those above, below, or within the reported efavirenz therapeutic range (1,000–4,000 ng/mL) and within subgroups.
Methods
This analysis examined efavirenz plasma concentrations obtained from participants enrolled in AIDS Clinical Trials Group Study A5202. This investigation divided subjects into those who experienced virologic failure and those who did not. These subjects were further separated to investigate those who had “high,” “within,” or “low” plasma concentrations, based on the therapeutic range. The association between virologic failure and plasma concentration was statistically examined in addition to the variables race/ethnicity, sex, assigned nucleos(t)ide reverse transcriptase inhibitor backbone, age at study entry, history of intravenous drug use, weight, and screening HIV-1 RNA stratification level.
Results
In univariate analyses, a statistically significant difference was found when comparing the efavirenz concentration groups, (22 failures among the “low” concentration group [19%], 65 failures among the “within” concentration group [12%], and 11 failures among the “high” concentration group [9%]) when evaluating virologic failure as an outcome (p = 0.04). In addition, the proportion of participants with virologic failure differed across race/ethnicity groups (p = 0.03) with black non-Hispanic participants observed to have the highest rate (17%). Efavirenz concentration group, race/ethnicity, age, weight, and the interaction between efavirenz concentration group and weight were found to be significantly associated with virologic failure in multivariable logistic regression analysis.
Conclusions
The proposed efavirenz therapeutic range, combined with the impact of a patient’s weight, is associated with virologic failure in HIV-infected ARV-naive individuals in the United States. Additional analysis is recommended to determine the most appropriate concentration value that defines the lower limit of the efavirenz therapeutic range.
Keywords: efavirenz, virologic failure, therapeutic range, therapeutic drug monitoring
Introduction
Efavirenz is a non-nucleoside reverse transcriptase inhibitor (NNRTI) commonly prescribed with nucleos(t)ide reverse transcriptase inhibitors (NRTIs) for combination use in the HIV-1 patient population. The United States Department of Health and Human Services Guidelines for the treatment of HIV-1 antiretroviral (ARV) naive patients suggest efavirenz as an alternative third drug agent.1 Globally, it is recommended as a preferred regimen combined with tenofovir disoproxil fumarate + lamivudine (or emtricitabine) when initiating ARV therapy, based upon moderate-quality evidence that this regimen was less frequently associated with severe adverse events and had improved efficacy when it was systematically compared to other NNRTI and PI regimens.2
In a study by Marzolini et al., efavirenz plasma concentrations were examined for the utility of prediction of HIV viral failure and central nervous system (CNS) adverse events.3 They found that patients with low (<1,000 ng/mL) and high (>4,000 ng/mL) mid-interval (average 14 hours after intake) efavirenz concentrations were more likely to experience virologic failure and CNS adverse events, respectively.3 When using population pharmacokinetic modeling, a possible relationship between viral load and average efavirenz concentrations was shown to exist.4 Therapeutic drug monitoring may be beneficial for certain conditions, such as in pregnant women, those with organ dysfunction, and those at risk for drug interactions5 to ensure that drug concentrations do not become subtherapeutic or reach toxic levels. The effect of genotypic differences on efavirenz concentrations, as well as the allele frequency of the major metabolizing enzyme in different race and ethnicity groups, has been described previously.6–8 Population pharmacokinetic modeling has shown that weight and fat-free mass affect the clearance of efavirenz; however, the association of sex with concentration differences appears to be inconclusive.8–10
AIDS Clinical Trials Group (ACTG) study A5202 was a study in HIV-1 treatment naive participants investigating initial treatment options.11–13 This study enrolled 1857 eligible participants and included the collection of drug concentration data in the majority, thus providing an opportunity to evaluate the therapeutic range utilized in the literature for efavirenz. We report on the comparison of participants who experienced virologic failure to those who did not in the efavirenz containing arms of ACTG study A5202 and efavirenz concentrations between these groups. The objective of this analysis was to compare virologic failure between those above, below, or within the reported efavirenz therapeutic range (1,000–4,000 ng/mL) and within subgroups.
Methods
Trial study design
Study A5202 was a phase IIIB randomized equivalence study to compare four ARV regimens in treatment-naive HIV-1-infected adults: atazanavir with ritonavir (300 mg/100 mg) or efavirenz (600 mg) and double-blinded, placebo-controlled NRTI backbone of abacavir/lamivudine (600 mg/300 mg) or tenofovir disoproxil fumarate/emtricitabine (300 mg/200 mg). Randomization was stratified by participants’ HIV-1 RNA level (<100,000 or ≥100,000 copies/mL) at screening, and participants were randomized 1:1:1:1 to one of the four treatment arms. The primary efficacy outcome was time from randomization to virologic failure, defined as a confirmed plasma HIV-1 RNA level ≥1000 copies/mL at or after 16 weeks and before 24 weeks or ≥200 copies/mL at or after 24 weeks after randomization.11 Results from the primary and main secondary analyses have been previously published.11–13
Drug concentration and HIV-1 RNA sampling
In study A5202, participants who received efavirenz in the evening were scheduled for two visits within the first 24 weeks of therapy to obtain three blood samples for drug concentrations—visits A and B. Visit A was to be scheduled around the observed dosing of the NRTI backbone. For four days prior to the visit, participants were asked to switch their NRTI dosing to the morning (but not their efavirenz dose due to toxicity concerns), and on the fifth day, two samples were to be obtained (~4 hours apart) around the dosing of the NRTI backbone. Visit B was planned to consist of an efavirenz plasma sample 5–15 hours after an efavirenz dose. Visit A could occur before or after visit B. A total of 3 efavirenz plasma samples were to be drawn within the first 24 weeks of therapy. The measurement of HIV-1 RNA was evaluated before study entry, at study entry, weeks 4, 16, 24, then every 12 weeks, at final study evaluation, evaluation after virologic failure was confirmed, and if there was a premature discontinuation of study treatment. Medication adherence training was provided at study entry. Self-reported adherence questionnaires were administered at weeks 8 and 24.
Efavirenz plasma concentrations were quantified by two ACTG-supported pharmacology laboratories utilizing validated high-performance liquid chromatography assays.14 The methods used to quantitate efavirenz employed reversed-phase high-performance liquid chromatography (RP-HPLC) using a photodiode-array detector scanning at 248 nm. The methods had a lower limit of quantitation of 0.1 μg/mL with an interday imprecision ranging from 3.6% to 5.4% (coefficients of variance, CV%) as determined based on the quality control samples. The laboratories participated in twice-annual proficiency testing to assure that the efavirenz results derived from the laboratories’ methods remained accurate.15 HIV-1 RNA measurement was performed for screening at any laboratory certified under the Clinical Laboratory Improvement Amendments and after screening at Johns Hopkins University.
Statistical analysis
The present study examined efavirenz concentrations from subjects enrolled in A5202, drawn between 14 and 190 days after initiating efavirenz. Subjects were further separated to investigate those that had “high” (at least one plasma concentration >4,000 ng/mL), “within” (all plasma concentrations within the range of 1,000 ng/mL–4,000 ng/mL), “low” (at least one plasma concentration <1,000 ng/mL), and “both” (at least one sample <1,000 ng/mL and one >4,000 ng/mL) plasma concentrations. Subjects were included in the analysis if they were taking efavirenz for at least 14 days prior to sampling, in order to ensure samples were collected at steady state.16 Additionally, efavirenz concentrations were used if the sample was drawn beyond 6.0 hours after efavirenz administration to ensure concentrations were collected beyond the absorption phase.16 Five samples were not included in the analysis because they had either unknown storage conditions or were drawn outside the limits of the protocol. Additionally, one sample (67 ng/mL) that was below the limit of quantification (100 ng/mL), but not 0 ng/mL, was removed from analysis; however, this did not change the efavirenz concentration group for this participant.
A5202 had a primary efficacy end point that considered time to virologic failure. In the present exploratory analysis, a binary outcome was utilized (virologic failure—Yes/No). Associations between virologic failure and covariates were tested using Fisher’s exact test for categorical or the Wilcoxon rank sum test for continuous covariates. The multivariable relationship between virologic failure and covariates was statistically assessed using logistic regression. Covariates included race/ethnicity, history of IV drug use, sex, NRTI backbone, age at study entry, screening HIV-1 RNA stratification level, first available weight measured during drug plasma sampling, and efavirenz plasma concentration group (“high,” “low,” “within,” and “both”). Furthermore, the interactions between the concentration grouping variable with race/ethnicity and first available weight during efavirenz sampling (categorized by quartile) and between race/ethnicity and sex were considered. Model fitting was implemented with R.17 Wald tests were performed to assess statistical significance.18 All tests were two-sided and tested at a 0.05 nominal significance level.
Three covariates (race/ethnicity, history of IV drug use, and efavirenz concentration group) had participant groups that consisted of a small number of participants. In order to address the computational problems this introduces when fitting the logistic regression, categories were combined as follows: race/ethnicity—Asian (n = 11), American Indian and Alaskan (n = 8), and >1 race (n = 5) were combined as “Other. Moreover, ” history of IV drug use “Currently” (n = 2) and “Previously” (n = 72) were combined, as well as efavirenz plasma concentration groups: “low” (n = 106) and “both” (n = 8) were combined since the present analysis was focused on the association of efavirenz concentrations and virologic failure rather than toxicity.
Results
A total of 929 study participants were randomized into the two efavirenz treatment arms, and 86% (n = 802) of these participants contributed a total of 2154 efavirenz drug concentration samples. Among these participants, 2000 efavirenz samples from 796 study participants met initial inclusion criteria for univariate analysis, and 784 participants contributed 1895 samples for the multivariable analysis (Figure 1). Figure 2 shows efavirenz concentrations for participants included in the current analysis, and these samples were collected within 14–190 days of beginning efavirenz. The majority of efavirenz samples were collected 10–15 hours post-dose in A5202.
Figure 1.
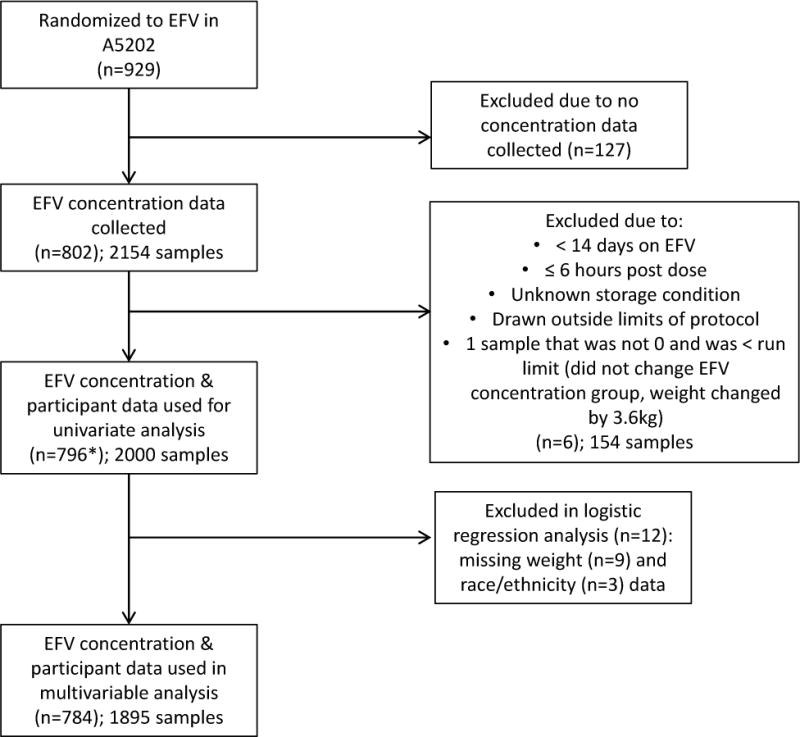
Number of participants included in the analysis.
Figure 2.
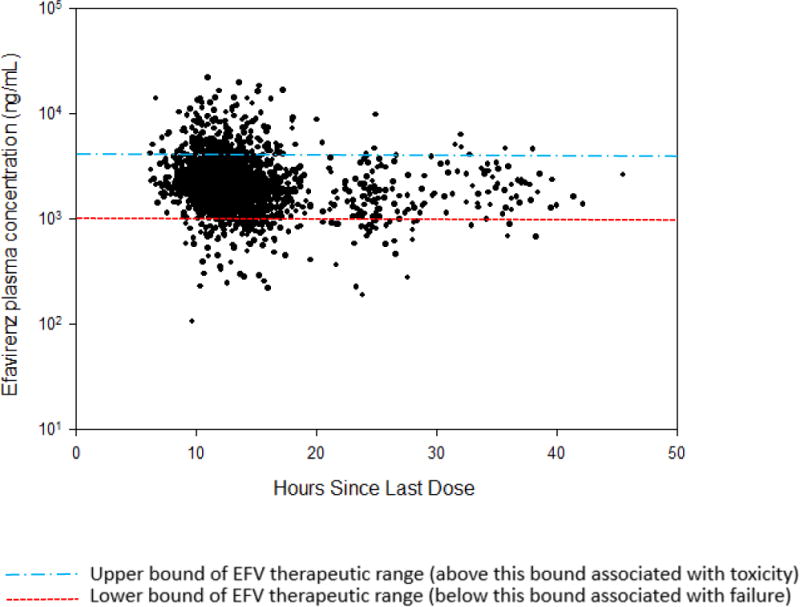
Efavirenz concentrations (observations = 2000) from participants of A5202 drawn within 14–190 days of beginning efavirenz.
Participant characteristics by virologic failure status are contained in Table 1. In univariate analyses, statistically significant differences were found when comparing the efavirenz concentration groups (22 failures among the “low” concentration group [19%], 65 failures among the “within” concentration group [12%], and 11 failures among the “high” concentration group [9%]), when evaluating virologic failure as an outcome (p = 0.04). In addition, the proportion of participants with virologic failure differed across race/ethnicity groups (p = 0.03) with black non-Hispanic participants observed to have the highest (17%). Lastly, there was a significant difference in the age distribution at study entry between those with virologic failure versus no failure (p = 0.01), as those who experienced virologic failure were slightly younger. All other sub-group comparisons showed non-significant differences when evaluating the outcome of virologic failure.
Table 1.
Characteristics of efavirenz participants by virologic failure status (Yes vs. No)
| Characteristics | Virologic failure (n = 98) |
No virologic failure (n = 698) |
p-Value for overall differences; Significance limit: p< 0.05 |
|---|---|---|---|
| Efavirenz Concentration Group, n (%) | |||
| Low¥ | 22 (19%) | 92 (81%) | 0.04 |
| Within | 65 (12%) | 493 (88%) | |
| High | 11 (9%) | 113 (91%) | |
| Assigned NRTIs, n (%) | |||
| TDF/FTC | 48 (12%) | 352 (88%) | 0.83 |
| ABC/3TC | 50 (13%) | 346 (87%) | |
| Sex, n (%) | |||
| Male | 83 (13%) | 570 (87%) | 0.57 |
| Median age, years (IQR) | 36 (29.8–43) | 38 (31–46) | 0.01 |
| Race/Ethnicity, n (%) | |||
| White, non-Hispanic | 31 (10%) | 283 (90%) | 0.03 |
| Black, non-Hispanic | 47 (17%) | 224 (83%) | |
| Hispanic | 18 (10%) | 166 (90%) | |
| Othera | 2 (8%) | 22 (92%)* | |
| Screening HIV-1 RNA, n (%) | |||
| ≥100,000 copies/mL | 44 (13%) | 296 (87%) | 0.66 |
| IV drug use, n (%) Never | 87 (12%) | 635 (88%) | 0.46 |
| Median weight, kg (IQR) € | 73.6 (65.6–86.3) | 76.4 (68.2–87.5) | 0.12 |
| Median weight, kg by Quartiles € Θ | |||
| Weight ≤ 1st Quartile | 62.36 | 62.05 | 0.74 |
| 1st Quartile < Weight ≤ Median | 72.27 | 71.82 | 0.96 |
| Median < Weight ≤ 3rd Quartile | 79.68 | 81.36 | 0.08 |
| Weight > 3rd Quartile | 95.50 | 97.50 | 0.30 |
NRTIs, nucleos[t]ide reverse transcriptase inhibitors; TDF/FTC, tenofovir disoproxil fumarate/emtricitabine; ABC/3TC, abacavir/lamivudine; IQR, interquartile range
11 Asian, 8 Native American/Alaskan Natives, 5 subjects reporting more than one race, and 3 subjects with missing race/ethnicity information
Low concentration group is combined with both concentration groups
Missing race/ethnicity values for three participants not included in the analysis (n = 25)
Missing weight values for nine participants not included in the analysis
Quartiles of the weight variable (kg rounded to the nearest whole number): 0% (40 kg), 25% (68 kg), 50% (76 kg), 75% (88 kg), 100% (172 kg)
In the multivariable logistic regression analysis, the covariates race/ethnicity (with “other,” white non-Hispanic, Hispanic, and black non-Hispanic being associated with an increasing probability of failure, with the white non-Hispanic population used as reference) and age (with increased age being associated with a decreasing probability of failure) were found to be statistically significant. Table 2 contains the corresponding odds ratios for covariates assessed in the logistic regression. The association of efavirenz plasma concentration groups with virologic failure depends on weight with the risk of virologic failure in the “low” group compared to the “within” or “high” efavirenz concentration groups being higher at lower weight (p = 0.036). Given that weight was treated as numeric in the multivariate analysis, Figure 3 displays the estimated odds ratios for group comparisons at the quartiles of the observed weight distribution, specifically at values 68, 76, and 87.7 kg.
Table 2.
Odds ratio table showing logistic regression associations with virologic failure
| Predictor | Estimated Odds ratio for virologic failure (95% CI) | P-value for overall differences Sig. < 0.05 |
|---|---|---|
| Race/ethnicity | ||
| Black, non-Hispanic vs. White, non-Hispanic | 2.2 (1.3, 3.8) | 0.002 |
| Hispanic vs. White, non-Hispanic | 1.05 (0.55, 2.0) | |
| Othera vs. White, non-Hispanic | 0.91 (0.14, 3.5) | |
| IV Drug Use | ||
| Current/Previous vs. Never | 1.5 (0.71, 3.1) | 0.19 |
| Sex | ||
| Female vs. Male | 0.7 (0.36, 1.3) | 0.18 |
| Assigned NRTIs | ||
| ABC/3TC vs. TDF/FTC | 1.02 (0.65, 1.6) | 0.91 |
| Age (every 5 years) | 0.86 (0.76, 0.96) | 0.002 |
| Screening HIV-1 RNA Stratification | ||
| ≥ 100,000 vs. < 100,000 copies/mL | 1.3 (0.83, 2.1) | 0.16 |
| Efavirenz Concentration Group & Weight Interactionb, c | ||
| Low vs. Within at 1st quartile (25%) of weight | 3.18 (1.60, 6.29) | 0.036 |
| Low vs. Within at median (50%) of weight | 2.28 (1.29, 4.04) | |
| Low vs. Within at 3rd quartile (75%) of weight | 1.41 (0.69, 2.89) | |
| Low vs. High at 1st quartile (25%) of weight | 5.41 (2.16, 13.57) | |
| Low vs. High at median (50%) of weight | 3.66 (1.59, 8.42) | |
| Low vs. High at 3rd quartile (75%) of weight | 2.07 (0.71, 6.08) |
NRTIs, nucleos[t]ide reverse transcriptase inhibitors; TDF/FTC, tenofovir disproxil fumurate/emtricitabine; ABC/3TC, abacavir/lamivudine.
11 Asian, 8 Native American/Alaskan Natives, 5 subjects reporting more than one race
Low efavirenz concentration group is combined with the both group
P-value from continuous version of the weight covariate.
Figure 3.
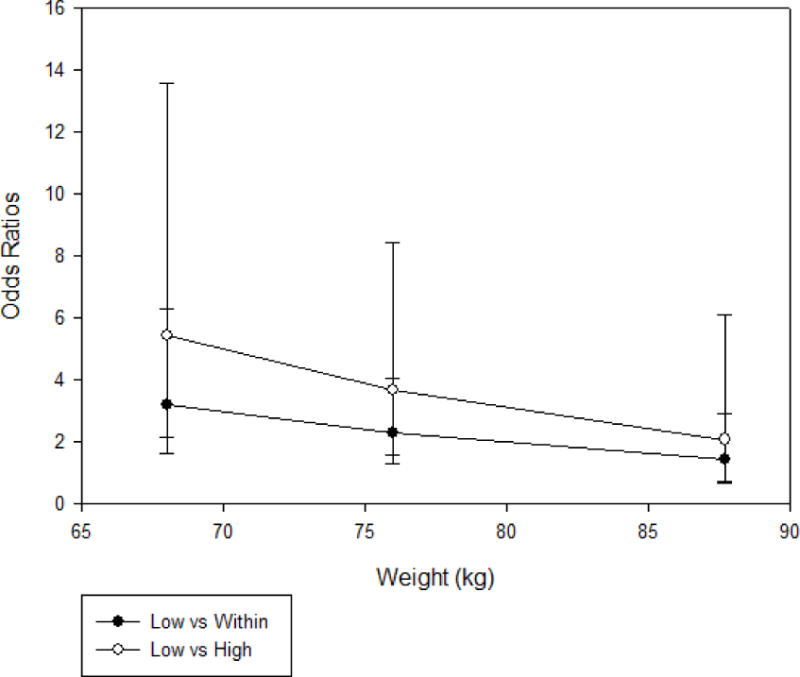
Estimated odds ratios for group comparisons at the quartiles of the observed weight distribution, specifically at the values 68, 76, and 87.7 kg.
Discussion
This secondary analysis of A5202 clinical trial data found that the association of efavirenz plasma concentrations with virologic failure depends on weight. A small number of subjects had measured efavirenz concentrations that fell into both the “high” and “low” category. For the necessity of the analysis, and based on the main objective of the study, this group was combined with those in the “low” group. The probability of virologic failure increased from “high,” “within,” and to “low” efavirenz concentration groups. While this suggests “high” levels were advantageous, this would need to be balanced against the potential for adverse effects, and this analysis did not look at the potential toxicity associated with high levels.
In the multivariable logistic regression, race/ethnicity, age at baseline, and the interaction term between efavirenz concentration group and weight were significantly associated with virologic failure. Sex was not significantly associated with virologic failure, which was primarily in agreement with a study of time to virologic failure in the A5202 study by Smith et al.19 In addition, there was no significant difference in outcome when evaluating history of IV drug use, the assigned NRTI backbone, and HIV-1 RNA stratification level at screening.
The association of efavirenz plasma concentration groups with virologic failure depends on weight, with the risk of virologic failure in the “low” group compared to the “within” or “high” efavirenz concentration groups being higher at lower weight. In a study by Marzolini et al. examining time to initial undetectable viral load in treatment-naive subjects on efavirenz, underweight subjects were significantly less likely to obtain an undetectable viral load when compared to their normal weight counterparts; however, this was not observed between heavier and normal-weight participants.20 In addition, the underweight group compared to normal weight had a significantly higher cumulative probability of virological rebound.20 One hypothesis that may explain the findings seen in the current study is that those who patients who were underweight were more likely to have toxicity associated with poorer adherence. To our knowledge, this relationship has not been examined in the literature. However, multivariate analysis (n = 41) indicated that efavirenz concentrations show a significant (p = 0.015) inverse relationship with body weight through.21
Younger age at study entry was associated with an increased probability of virologic failure, the average (range) was 36 (18–58) and 39 (18–69), between those experiencing virologic failure and those not. Others have reported younger age as an independent predictor of virologic failure in HIV-infected patients on NNRTI-based regimens.22 Although in our population there was only a three-year mean difference between those with virologic failure and non-failure, there was a significant shift in the distribution. A difference was also observed when comparing virologic failure and non-failure among race/ethnicity groups. The probability of virologic failure in the current analysis was greatest for black non-Hispanic participants. This was also shown in a separate analysis that included A5202 data for subjects who had confirmed virologic failure and pretreatment and failure gene sequence results (265/269), in which the most common race/ethnicity was those who were black non-Hispanic.23 In the current study, within the virologic failure group, 26% of white non-Hispanic subjects experienced at least one concentration out of the therapeutic range, with black non-Hispanic subjects experiencing 43% (20/47). Among the black non-Hispanic subjects with an out-of-range efavirenz concentration, 40% (n = 8) had at least one concentration above the therapeutic range and 55% (n = 11) with at least one below the range, with 5% (n = 1) experiencing both above and below. Compared to white non-Hispanic, those who were black non-Hispanic have been associated with an increased risk of virologic failure in adjusted analyses.24 Furthermore, a greater impact of non-adherence on virologic failure has been reported for black versus white subjects on an efavirenz-containing regimen for the initial treatment of HIV infection.25 In a genome-wide association study, involving multiple ACTG studies, including A5202, three CYP2B6 polymorphisms were found to be independently associated with efavirenz Cmin (minimum plasma concentration).7 However, predictive genetic variants of efavirenz exposure were examined in a study that included A5202 data for those related to the lowest efavirenz plasma exposure and none were found to have a significant association with virologic failure.26 In addition, the study found no genome-wide significant associations with virologic response for efavirenz.26
A therapeutic range for efavirenz between 1,000 and 4,000 ng/mL for mid-dosing interval samples has been utilized previously.27,28 However, studies such as ENCORE1, which compared the efficacy of 400 mg of efavirenz to the standard dose of 600 mg at 48 and 96 weeks in treatment-naive patients, suggest that 1,000 ng/mL does not necessarily represent the optimal efficacy concentration cutoff based on the proportion of subjects with both a plasma viral load level ≥200 copies/mL and predicted efavirenz mid-dosing concentrations <1,000 ng/mL compared to >1,000 ng/mL (p = 0.059).29,30 However, caution is recommended in interpretation based on the limited number of virologic failures and projection of pharmacokinetic parameters to a pharmacodynamic outcome at a much later time point.30 In addition, a range of possible minimum effective concentrations were suggested (470–760 ng/mL), but additional analysis would be required to confirm this.30 Other studies have reported no correlation between viral suppression and efavirenz exposure.31 In a study comparing the efficacy of three-times-a-week dosing to daily dosing of the combined antiretroviral medication efavirenz, emtricitabine, and tenofovir disoproxil fumarate in HIV-1 patients, with no history of virologic failure or resistance mutations to study drugs, all subjects (in both arms) maintained HIV-1 RNA <37 copies/mL for 24 weeks. In this study, the three-day-per-week group had significantly lower efavirenz concentrations (plasma concentrations were measured 12 hours after the dose in the daily treatment arm and 60 hours after the dose in the three-times-a-week arm).32
Overall, this study found that in addition to plasma concentrations and weight, race/ethnicity and age at study entry may be significantly associated with virologic failure. In addition, the association of efavirenz plasma concentration groups with virologic failure depends on weight with the risk of virologic failure in the “low” group compared to the “within” or “high” efavirenz concentration groups being increased at lower weight.
Limitations to the current study include unknown reasons for sub-therapeutic levels, such as possible drug–drug interactions, non-adherence, comorbid conditions, or other unmeasured confounding factors. Although a mean intra-patient variability of about 30% (coefficient of variation (CV)) on repeated efavirenz plasma concentration measurements over approximately three-month intervals has been reported,3,33 the current study assumes that an efavirenz concentration drawn within six months of beginning therapy may affect an outcome at a time point later than sampling, as the original study was continued for a duration lasting 96 weeks after the enrollment of the last participant. As the current analysis evaluated sparse plasma samples collected within the first 190 days of therapy, the potential limitation of unknown adherence is recognized, as adherence can vary widely over time and across a population. Additionally, we did not evaluate the opposite end of the recommended therapeutic range of efavirenz (>4000 ng/mL), which is associated with increased risk of toxicity, which could possibly lead to decreased adherence and may ultimately result in virologic failure.
Conclusion
As decreased efavirenz dosing is further examined for use on a global basis to decrease cost and adverse effects while maintaining efficacy, additional analysis is recommended to determine the most appropriate concentration value to define the lower limit of the efavirenz therapeutic range in varied populations. This future direction could help determine the absolute cutoff associated with efficacy, to aid in improving the outcome of ART around the globe.
Acknowledgments
Other investigators and contributors included the following
Hector H. Bolivar, MD, and Sandra Navarro, MD—University of Miami (Site 901) CTU Grant #AI069477, ACTG Grant #AI27675, CFAR Grant #AI073961; Susan L. Koletar, MD, and Diane Gochnour, RN—Ohio State University (Site 2301) CTU Grant #AI069474; Edward Seefried, RN, and Julie Hoffman, RN—University of California, San Diego (Site 701) CTU Grant #AI69432; Judith Feinberg, MD, and Michelle Saemann, RN—University of Cincinnati (Site 2401) CTU Grant #AI069513; Kristine Patterson, MD, Donna Pittard, RN, and David Currin, RN—University of North Carolina (Site 3201) CTU Grant #AI69423, CFAR Grant #AI50410, GCRC Grant #RR00046, and Grant #RR025747; Kerry Upton, RN, BSN, and Michael Saag, MD—University of Alabama (Site 5801) CTU Grant #U01 AI069452, CCTS Grant #1UL1 RR025777-01; Graham Ray and Steven Johnson—University of Colorado Health Sciences Center (Site 6101) CTU Grant #AI69450, Grant #AI054907, and Grant #RR00051; Bartolo Santos, RN, and Connie A. Funk, RN, MPH—University of Southern California (Site 1201) CTU Grant #5U01 AI069428; Michael Morgan, FNP, and Brenda Jackson, RN—Vanderbilt Therapeutics CRS (Site 3652) CTU Grant #AI069439; Pablo Tebas, MD, and Aleshia Thomas, RN—University of Pennsylvania, subunit of Children’s Hospital of Philadelphia (Site 6201) CTU Grant #U01 AI069467-03, CFAR Grant #5P30 AI045008-10; Ge-Youl Kim, RN, BSN, and Michael K. Klebert, PhD, RN, ANP-BC—Washington University (Site 2101) CTU Grant #AI069495; Jorge L. Santana and Santiago Marrero—University of Puerto Rico (Site 5401) CTU Grant #5U01 AI069415-03; Jane Norris, PA-C and Sandra Valle, PA-C—Stanford University (Site 501) CTU Grant #AI69556; Gary Matthew Cox, MD, and Martha Silberman, RN—Duke University Medical Center (Site 1601) CTU Grant #5U01 AI069484-02; Sadia Shaik and Ruben Lopez—Harbor-University of California, Los Angeles Medical Center (Site 603) CTU Grant #AI069424; Margie Vasquez, RN, and Demetre Daskalakis, MD—New York University/NYC HHC at Bellevue Hospital Center (Site 401) CTU Grant #AI069532; Christina Megill, RPA-C, and Todd Stroberg, RN—Cornell Chelsea (Site 7804) CTU Grant #AI69419, CSTC Grant #RR024996; Jessica Shore, BSN, and Babafemi Taiwo, MBBS—Northwestern University CRS (Site 2701) CTU Grant #AI069471; Mitchell Goldman, MD, and Molly Boston, RN—Indiana University (Site 2601) CTU Grant #UO1 AI025859; Dr. Jeffrey Lennox and Dr. Carlos del Rio—Ponce de Leon Center (A5802) CTU Grant #5U01 AI069418, CFAR Grant #P30AI050409; Timothy W. Lane, MD, and Kim Epperson, RN—Moses H. Cone Memorial Hospital (Site 3203) CTU Grant #1U01 A1069423-01; Annie Luetkemeyer, MD, and Mary Payne, RN—University of California, San Francisco (Site 801) CTU Grant #1U01 AI069502-01; Barbara Gripshover, MD, and Dawn Antosh, RN—Case Western Reserve University (Site 2501) CTU Grant #AI69501; Jane Reid, RN, MS, APN-BC, and Mary Adams, RN, MPH—University of Rochester (Site 1101) CTU Grant #U01 AI069511, GCRC Grant #UL1 RR024160; Sheryl S. Storey, PA-C, and Shelia B. Dunaway, MD—University of Washington (Site 1401) CTU Grant #AI069434; Joel Gallant, MD, and Ilene Wiggins, RN—Johns Hopkins University (Site 201) CTU Grant #AI69465; Kimberly Y. Smith, MD, MPH, and Joan A. Swiatek, RN, APN—Rush University Medical Center (Site 2702) CTU Grant #5U01 AI069471; Joseph Timpone, MD, and Princy Kumar, MD—Georgetown University (Site 1008) CTU Grant #1U01 AI069494-01; Ardis Moe, MD, and Maria Palmer, PA-C—University of California, Los Angeles Care Center (Site 601) CTU Grant #AI069424; Jon Gothing, RN, BSN, ACRN, and Joanne Delaney, RN, BSN—Brigham and Women’s Hospital, Boston, MA (Site 107) CTU Grant #AI069472; Kim Whitely, RN, and Ann Marie Anderson, RN—Metro Health Center (Site 2503) CTU Grant #AI069501; Scott M. Hammer and Michael T. Yin—HIV Prevention & Treatment (Columbia University) (Site 30329) CTU Grant #5U01 AI069470, Grant #1UL1 RR024156; Mamta Jain, MD, and Tianna Petersen, MS—UT Southwestern Medical Center at Dallas (Site 3751) CTU Grant #3U01 AI046376 05S4; Roberto Corales, DO, and Christine Hurley, RN—AIDS Community Health Center (Site 1108) CTU Grant #U01AI069511, GCRC Grant #UL1 RR024160; Keith Henry, MD, and Bette Bordenave, RN—Hennepin County Medical Center (Site 1502) Grant #N01 AI72626; Amanda Youmans, NP, and Mary Albrecht, MD—Beth Israel Deaconess (Partners/Harvard) CRS (Site 103) CTU Grant #UOI A106947203; Richard B. Pollard, MD, and Abimbola Olusanya, NP—University of California, Davis Medical Center (Site 3851) Grant #AI38858; Paul R. Skolnik, MD, and Betsy Adams, RN—Boston Medical Center CRS (Site 104) CTU Grant #AI069472; Karen T. Tashima and Helen Patterson—Miriam Hospital-Brown University (Partners/Harvard) (Site 2951) CTU Grant #1U01 AI069472-01; Michelle Ukwu and Lauren Rogers—Peabody Health Center (Site 31443) CTU Grant #AI069471; Henry H. Balfour, Jr., MD, and Kathy A. Fox, RN, MBA—University of Minnesota (Site 1501) CTU Grant #AI27661; Susan Swindells, MBBS, and Frances Van Meter, APRN—University of Nebraska Medical Center (Site 1505) CTU Grant #AI27661; University of Hawaii (Site 5201) CTU Grant #AI34853; Gregory Robbins, MD, and Nicole Burgett-Yandow, RN, BSN—Massachusetts General Hospital from the Partners/Harvard/BMC ACTU (Site 101) CTU Grant #1U01 AI069472-01; Dr. Charles E. Davis, Jr. and Colleen Boyce, RN—IHV Baltimore Treatment CRS (Site 4651) CTU Grant #5U01 AI069447 03; William A. O’Brien, MD, and Gerianne Casey—University of Texas Medical Branch (Site 6301) CTU Grant #AI032782; Dr. Gene D. Morse, PharmD, and Dr. Chiu-Bin Hsaio, MD—SUNY-Buffalo (Site 1102) CTU Grant #5U01 A1027658; San Mateo County AIDS Program (Site 505) CTU Grant #AI27666; Jeffrey L. Meier and Jack T. Stapleton—University of Iowa Healthcare (Site 1504) NIAID Grant #AI27661, Grant #AI58740; Donna Mildvan, MD, and Manuel Revuelta, MD—Beth Israel Medical Center ACTU (Site 2851) CTU Grant #AI46370; David Currin, RN—Wake County HHS (Site 30076) CTU Grant #AI25868; Wafaa El Sadr, MD, MPH, MPA and Avelino Loquere, RN—Harlem ACTG CRS (Site 31483) CTU Grant #5U01 AI069470-03; Nyef El-Daher, MD and Tina Johnson, RN—McCree McCuller Wellness Center (Site 1107) CTU Grant #U01 AI069511, GCRC Grant #UL1 RR024160; Robert Gross MD, MSCE and Kathyrn Maffei, RN, BSN—University of Pennsylvania Health (Site 6206) CTU Grant #1U01 AI69467-01; Valery Hughes, FNP and Glenn Sturge, BS—Cornell Uptown (Site 7803) CTU Grant #1U01 AI069419-01; Deborah McMahon, MD and Barbara Rutecki, CRNP, MPH—University of Pittsburgh (Site 1001) CTU Grant #1UO1 AI069494-01; Michael Wulfsohn, MD PhD, Andrew Cheng, MD PhD and Norbert Bischofberger PhD—Gilead Sciences; and Lynn Dix, PhD and Qiming Liao, PhD—GlaxoSmithKline, Inc.
Research reported in this publication was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award Number UM1 AI068634, UM1 AI068636, U01 AI068636-01, UM1 AI069481, AI027757, P30 AI022763, and UM1 AI106701 and by the National Center for Advancing Translational Sciences of the National Institutes of Health under award Number UL1TR001412. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. CV and QM were supported in part by K23AI108355 and K08MH098794, respectively.
K.S. is an employee if ViiV Healthcare, beginning December 2013. ESD’s institution receives grant support from Gilead, Merck, and ViiV, and he is a consultant for Bristol Myers Squibb, Gilead, Merck, Janssen, Teva, ViiV, and Theratechnologies. ACC is a member of a Data and Safety Monitoring Board for a Merck-sponsored study, and her institution has received grant support from Bristol-Myers-Squibb. PES: Consultant or Scientific Advisory Board member: AbbVie, BMS, Gilead, GSK/ViiV, Merck, Janssen. Grant support to institution for research: BMS, Gilead, Merck, GSK/ViiV.
Footnotes
Conflict of Interest
The other authors have no conflict of interest to declare.
References
- 1.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. What to Start: Initial Combination Regimens for the Antiretroviral-Naive Patient. Available at http://aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf. Accessed: May 27, 2016.
- 2.WHO. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. 2nd. Geneva: World Health Organization; Jun, 2016. Available at: http://www.who.int/hiv/pub/arv/arv-2016/en/. Accessed: November 17, 2016. [PubMed] [Google Scholar]
- 3.Marzolini C, Telenti A, Decosterd LA, et al. Efavirenz plasma levels can predict treatment failure and central nervous system side effects in HIV-1-infected patients. AIDS. 2001;15:71–75. doi: 10.1097/00002030-200101050-00011. [DOI] [PubMed] [Google Scholar]
- 4.Csajka C, Marzolini C, Fattinger K, et al. Population pharmacokinetics and effects of efavirenz in patients with human immunodeficiency virus infection. Clin Pharmacol Ther. 2003;73:20–30. doi: 10.1067/mcp.2003.22. [DOI] [PubMed] [Google Scholar]
- 5.Gunthard HF, Aberg JA, Eron JJ, et al. Antiretroviral treatment of adult HIV infection: 2014 recommendations of the International Antiviral Society-USA Panel. JAMA. 2014;312:410–425. doi: 10.1001/jama.2014.8722. [DOI] [PubMed] [Google Scholar]
- 6.Naidoo P, Chetty VV, Chetty M. Impact of CYP polymorphisms, ethnicity and sex differences in metabolism on dosing strategies: the case of efavirenz. Eur J Clin Pharmacol. 2014;70:379–389. doi: 10.1007/s00228-013-1634-1. [DOI] [PubMed] [Google Scholar]
- 7.Holzinger ER, Grady B, Ritchie MD, et al. Genome-wide association study of plasma efavirenz pharmacokinetics in AIDS Clinical Trials Group protocols implicates several CYP2B6 variants. Pharmacogenet Genomics. 2012;22:858–867. doi: 10.1097/FPC.0b013e32835a450b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robarge JD, Metzger IF, Lu J, et al. Population pharmacokinetic modeling to estimate the contributions of genetic and nongenetic factors to efavirenz disposition. Antimicrob Agents Chemother. 2017;61:e01813–16. doi: 10.1128/AAC.01813-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arab-Alameddine M, Di Iulio J, Buclin T, et al. Pharmacogenetics-based population pharmacokinetic analysis of efavirenz in HIV-1-infected individuals. Clin Pharmacol Ther. 2009;85:485–494. doi: 10.1038/clpt.2008.271. [DOI] [PubMed] [Google Scholar]
- 10.Burger D, van der Heiden I, la Porte C, et al. Interpatient variability in the pharmacokinetics of the HIV non-nucleoside reverse transcriptase inhibitor efavirenz: the effect of gender, race, and CYP2B6 polymorphism. Br J Clin Pharmacol. 2006;61:148–154. doi: 10.1111/j.1365-2125.2005.02536.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sax PE, Tierney C, Collier AC, et al. Abacavir-lamivudine versus tenofovir-emtricitabine for initial HIV-1 therapy. New Engl J Med. 2009;361:2230–2240. doi: 10.1056/NEJMoa0906768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Daar ES, Tierney C, Fischl MA, et al. Atazanavir plus ritonavir or efavirenz as part of a 3-drug regimen for initial treatment of HIV-1. Ann Intern Med. 2011;154:445–456. doi: 10.1059/0003-4819-154-7-201104050-00316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sax PE, Tierney C, Collier AC, et al. Abacavir/lamivudine versus tenofovir DF/emtricitabine as part of combination regimens for initial treatment of HIV: final results. J Infect Dis. 2011;204:1191–1201. doi: 10.1093/infdis/jir505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keil K, Frerichs VA, DiFrancesco R, et al. Reverse phase high-performance liquid chromatography method for the analysis of amprenavir, efavirenz, indinavir, lopinavir, nelfinavir and its active metabolite (M8), ritonavir, and saquinavir in heparinized human plasma. Ther Drug Monit. 2003;25:340–346. doi: 10.1097/00007691-200306000-00015. [DOI] [PubMed] [Google Scholar]
- 15.DiFrancesco R, Tooley K, Rosenkranz SL, et al. Clinical pharmacology quality assurance for HIV and related infectious diseases research. Clin Pharmacol Ther. 2013;93:479–482. doi: 10.1038/clpt.2013.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Atripla (efavirenz/emtricitabine/tenofovir) [Prescirbing Information] Bristol-Myers Squibb & Gilead. 2015 Jan; [Google Scholar]
- 17.R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2016. Available at: https://www.R-project.org/ [Google Scholar]
- 18.Hosmer DW, Lemeshow S. Applied Logistic Regression. New York: Wiley and Sons; 2000. [Google Scholar]
- 19.Smith KY, Tierney C, Mollan K, et al. Outcomes by sex following treatment initiation with atazanavir plus ritonavir or efavirenz with abacavir/lamivudine or tenofovir/emtricitabine. Clin Infect Dis. 2014;58:555–563. doi: 10.1093/cid/cit747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marzolini C, Sabin C, Raffi F, et al. Impact of body weight on virological and immunological responses to efavirenz-containing regimens in HIV-infected, treatment-naive adults. AIDS. 2015;29:193–200. doi: 10.1097/QAD.0000000000000530. [DOI] [PubMed] [Google Scholar]
- 21.Poeta J, Linden R, Antunes MV, et al. Plasma concentrations of efavirenz are associated with body weight in HIV-positive individuals. J Antimicrob Chemother. 2011;66:2601–2604. doi: 10.1093/jac/dkr360. [DOI] [PubMed] [Google Scholar]
- 22.Parienti JJ, Massari V, Descamps D, et al. Predictors of virologic failure and resistance in HIV-infected patients treated with nevirapine- or efavirenz-based antiretroviral therapy. Clin Infect Dis. 2004;38:1311–1316. doi: 10.1086/383572. [DOI] [PubMed] [Google Scholar]
- 23.Mollan K, Daar ES, Sax PE, et al. HIV-1 amino acid changes among participants with virologic failure: associations with first-line efavirenz or atazanavir plus ritonavir and disease status. J Infect Dis. 2012;206:1920–1930. doi: 10.1093/infdis/jis613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ribaudo HJ, Smith KY, Robbins GK, et al. Racial differences in response to antiretroviral therapy for HIV infection: an AIDS clinical trials group (ACTG) study analysis. Clin Infect Dis. 2013;57:1607–1617. doi: 10.1093/cid/cit595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schackman BR, Ribaudo HJ, Krambrink A, et al. Racial differences in virologic failure associated with adherence and quality of life on efavirenz-containing regimens for initial HIV therapy: results of ACTG A5095. J Acquir Immune Defic Syndr. 2007;46:547–554. doi: 10.1097/qai.0b013e31815ac499. [DOI] [PubMed] [Google Scholar]
- 26.Lehmann DS, Ribaudo HJ, Daar ES, et al. Genome-wide association study of virologic response with efavirenz-containing or abacavir-containing regimens in AIDS clinical trials group protocols. Pharmacogenet Genomics. 2015;25:51–59. doi: 10.1097/FPC.0000000000000106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fayet Mello A, Buclin T, Decosterd LA, et al. Successful efavirenz dose reduction guided by therapeutic drug monitoring. Antivir Ther. 2011;16:189–197. doi: 10.3851/IMP1742. [DOI] [PubMed] [Google Scholar]
- 28.Meng X, Yin K, Wang J, et al. Effect of CYP2B6 gene polymorphisms on efavirenz plasma concentrations in Chinese patients with HIV infection. PLoS One. 2015;10:e0130583. doi: 10.1371/journal.pone.0130583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dickinson L, Amin J, Else L, et al. Pharmacokinetic and pharmacodynamic comparison of once-daily efavirenz (400 mg vs. 600 mg) in treatment-naive HIV-infected patients: results of the ENCORE1 study. Clin Pharmacol Ther. 2015;98:406–416. doi: 10.1002/cpt.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dickinson L, Amin J, Else L, et al. Comprehensive pharmacokinetic, pharmacodynamic and pharmacogenetic evaluation of once-daily efavirenz 400 and 600 mg in treatment-naive HIV-infected patients at 96 weeks: results of the ENCORE1 study. Clin Pharmacokinet. 2016;55:861–873. doi: 10.1007/s40262-015-0360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mukonzo JK, Owen JS, Ogwal-Okeng J, et al. Pharmacogenetic-based efavirenz dose modification: suggestions for an African population and the different CYP2B6 genotypes. PLoS One. 2014;9:e86919. doi: 10.1371/journal.pone.0086919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martínez E, Rojas J, JBlanco JL, et al. Three-day Per Week Atripla® in Patients with Sustained Viral Suppression. American Society of Microbiology Microbe; Boston MA USA: 2016. Jun 16–20, [Google Scholar]
- 33.Pereira SA, Branco T, Caixas U, et al. Intra-individual variability in efavirenz plasma concentrations supports therapeutic drug monitoring based on quarterly sampling in the first year of therapy. Ther Drug Monit. 2008;30:60–66. doi: 10.1097/FTD.0b013e318160ce76. [DOI] [PubMed] [Google Scholar]


