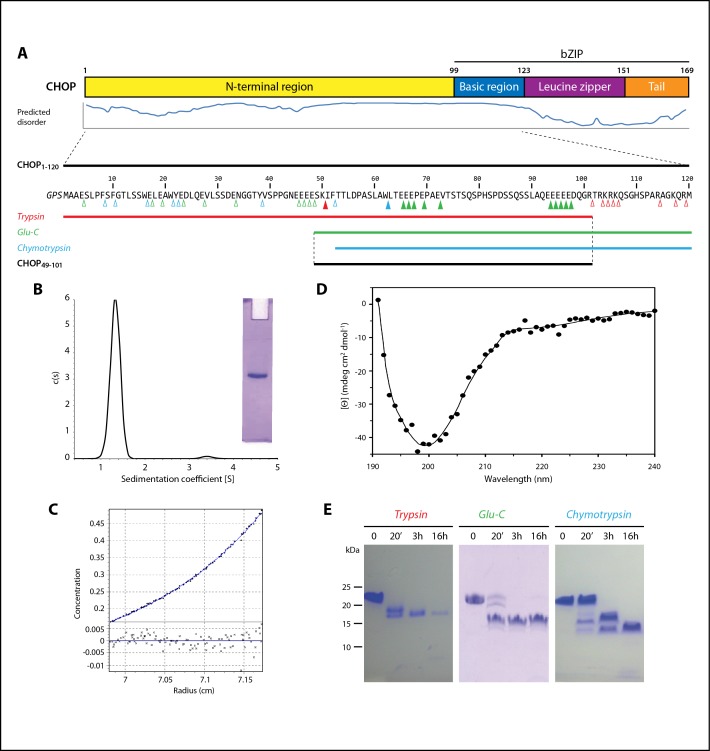
Fig 1. Biochemical characterization of the intrinsically disordered regions of CHOP.
(A) Bar diagram of CHOP domains with level of disorder according to Disopred [15]. Below, sequence of our CHOP1-120 construct with red, green and blue triangles marking possible cleavage sites for trypsin, Glu-C and chymotrypsin, respectively. Empty and filled triangles indicate protease-accessible and protease-protected positions, respectively. Coloured bars represent the fragments resulting from proteolysis experiments, while the bottom black bar shows the fragment obtained from the combined action of trypsin and Glu-C. (B) Sedimentation velocity and native PAGE (inset), of CHOP1-120, showing that the sample is monodisperse. (C) Sedimentation equilibrium of the same sample. (D) Circular dichroism spectrum of CHOP1-120. Experimental measurements of the sample after subtraction of the buffer signal are shown as dots, with an smoothen curve as delivered by Dichroweb (http://dichroweb.cryst.bbk.ac.uk/). (E) Time-course proteolytic digestion of CHOP1-120 as seen by Tris-tricine PAGE.