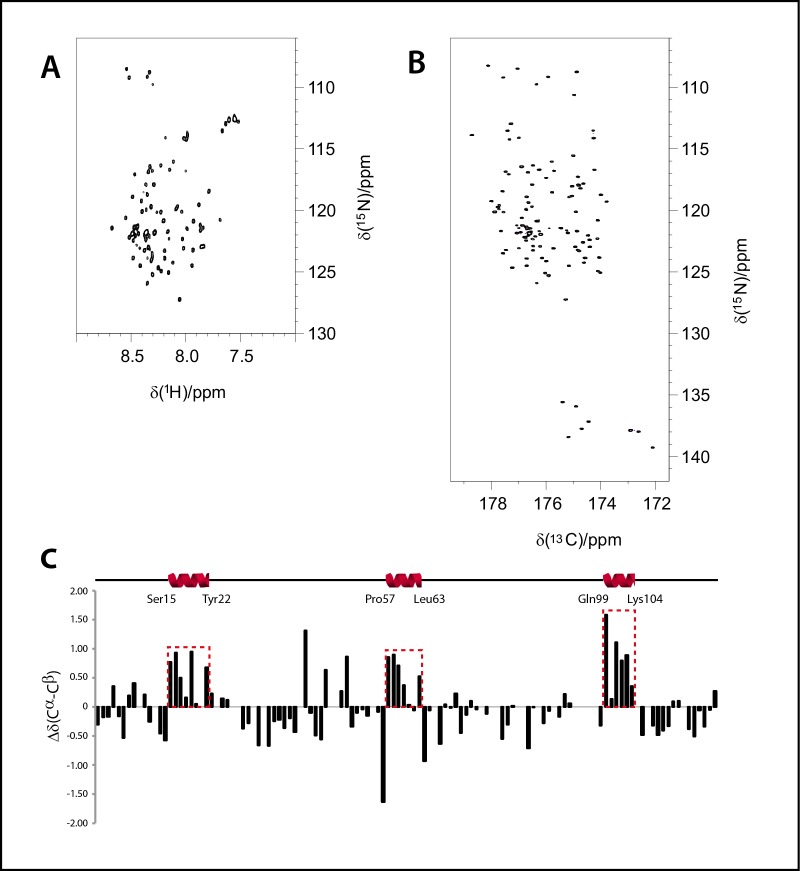
Fig 3. α-helical segments in the intrinsically disordered regions of CHOP.
(A) 1H-15N HSQC spectrum of our CHOP1-120 construct. (B) CON spectrum of the same sample. (C) Plots of the observed differences in experimental chemical shifts (Cα–Cβ) with respect to those predicted for a random coil. The data have been corrected by considering the contribution of the corresponding primary sequence, using random coil chemical shifts for intrinsically disordered proteins (http://www1.bio.ku.dk/english/research/bms/research/sbinlab/groups/mak/randomcoil/script/). A schematic representation of the segments with α-helical propensity is shown above.