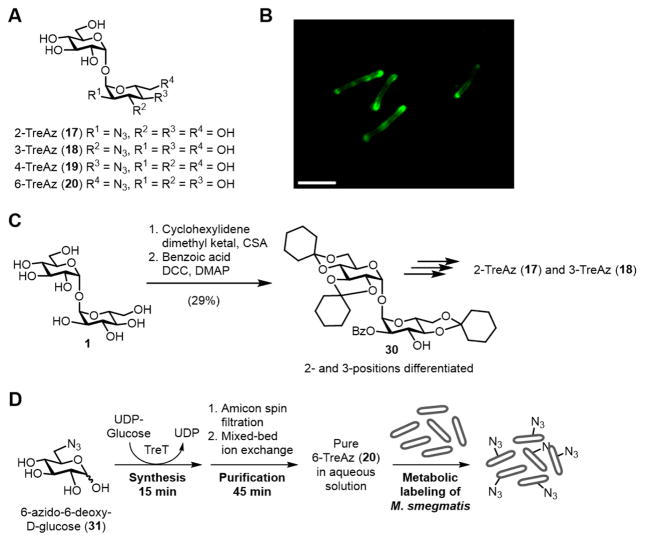
Figure 10.
(A) Structures of 2-, 3-, 4-, and 6-TreAz (17–20). (B) Metabolic labeling of M. smegmatis with TreAz followed by CuAAC with an alkyne-modified fluorophore (image reprinted with permission from ref. 100). Scale bar, 5 μm. (C) An example of the approach used to synthesize TreAz analogues. Differentiation of the 2- and 3-O-positions of trehalose (1) was accomplished in two steps, giving an intermediate (30), which was elaborated to 2- and 3-TreAz (17 and 18). (D) Rapid TreT-catalyzed synthesis, purification, and administration of 6-TreAz (20) to M. smegmatis for a metabolic labeling experiment. CSA, camphor sulfonic acid; DCC, N,N’-dicyclohexylcarbodiimide; DMAP, 4-dimethylaminopyridine.