Abstract
Objective
This literature review and meta-analysis was performed to evaluate the correlations among hearing and vestibular clinical symptoms, temporal bone findings, and pathological mechanisms in patients with systemic lupus erythematosus (SLE).
Study design
Relevant papers in the literature were retrospectively reviewed. Clinical hearing aspects in patients with SLE and relevant temporal bone studies in the same field were analyzed.
Methods
PubMed and Google Scholar searches were performed using the following keywords: “auto-immune disease,” “systemic lupus erythematosus (SLE),” “hearing loss,” “temporal bone study,” “vertigo,” “dizziness,” “tinnitus,” “ear symptoms,” “treatment,” “diagnosis,” “symptoms,” “etiopathogenesis,” “Wegener granulomatosis,” “Sjogren,” “polyarteritis nodosa,” “Cogan syndrome,” and “granulomatosis.” Also included were reviews in which the following terms were present: “SLE,” “temporal bone,” and “hearing symptoms.”
Review and conclusion
This literature review and meta-analysis focused on the pathological mechanisms through which SLE can damage inner ear structures and determinate hearing and vestibular symptoms. The main mechanisms involved in inner ear damage include the autoimmune response, deposition of immune complexes in the vessels and, to a lesser extent, cytotoxic damage.
Keywords: Systemic lupus erythematosus, hearing loss, tinnitus, dizziness, autoimmune disease
Introduction
Systemic lupus erythematosus (SLE) is an autoimmune disease with multiorgan involvement and an incidence of 12.5–39.0 per 100,000 people in the general population. The incidence of SLE is higher in women (82%–96%) than in men (4%–18%),1–2 and it is two to three times more prevalent in people of African and Asian descent than those of European one.3 Onset is most frequent from 20 to 39 years of age.4
SLE is a multifactorial pathology with different aetiologies, including genetic chromosome alterations, inflammation, drugs, environmental factors, and interactions between the adaptive and innate immune systems.1–3
The hallmark of SLE is the production of autoantibodies that react with self-nuclear and cytoplasmic antigens and culminate in immunologic attacks on body organs, resulting in tissue inflammation and multiorgan damage.4 T- and B-lymphocyte disorder plays a central role in this autoimmune dysfunction.5 The role of natural killer T cells has also been explored.6,7 Autoantibodies are directed toward antigens at the nuclear cell level; one of the most relevant antigens in SLE is double-stranded DNA. Antibody-mediated attacks \ primarily involve tissues and cells; however, they also target the walls of blood vessels, resulting in a generalized vascular alteration defined as vasculitis.8
As a systemic disease, SLE can involve different organs including the skin, kidney, neurologic system, and musculoskeletal system. Recent studies have also shown involvement of the inner ear.9 The aim of this study was to review the current literature with respect to hearing disorders in patients with SLE and evaluate the correlations among the pathophysiology, clinical symptoms, and temporal bone findings of SLE.
Material and methods
A systematic review was conducted by searching PubMed and Google Scholar. The following keywords were used to identify relevant articles: “autoimmune disease,” systemic lupus erythematosus (SLE),” “hearing loss,” “temporal bone study,” “vertigo,” “dizziness,” “tinnitus,” “ear symptoms,” “treatment,” “diagnosis,” “symptoms,” “etiopathogenesis,” “Wegener granulomatosis,” “Sjogren,” “polyarteritis nodosa,” “Cogan syndrome,” and “granulomatosis.” Studies in which the following words were present were included in the review: “SLE,” “temporal bone,” and “hearing symptoms.”
Table 1 summarizes the collected data for each publication selected for inclusion in the review. In total, 49 articles were reviewed.
Table 1.
Study collection data.
| Author | Audiogram |
|---|---|
| Type of article | Type of hearing loss |
| Year of publication | Cytocochleogram |
| Number of patients | Description of bone preparation |
| Systemic diseases | Involved ear structures |
| Therapy used | Middle ear aspect |
| Otologic symptoms | Inner ear aspect |
Audiovestibular disorders in patients with SLE
Clinical findings
SLE is characterized by the participation of multiple antibodies in immune-mediated tissue injury in different tissues and organs, including the auditory system. At this level, there are several mechanisms by which antibodies may damage the inner ear: 1) humoral-type antibody attacks on inner ear antigens, 2) cell-mediated cytotoxic damage to cochlear and vestibular hair cells, and 3) immune-complex deposition in the microvessels of the inner ear.5
The most common otologic symptom found in clinical studies of patients with SLE is sensorineural hearing loss (SNHL), the reported prevalence of which ranges from 6% to 70%.10–16 Table 2 summarizes the different subtypes of hearing loss found in patients with SLE in multiple studies performed from 1995 to 2013 (Table 2). Various authors have reported different pathways of SNHL in patients affected by SLE: hearing loss may either be slowly progressive or acute. It mainly affects high frequencies, mimicking the typical presbycusis pattern; however, it may also affect the low and middle frequencies. Maciaszczyk et al.10 described progressive SNHL involving all frequencies except 500, 2000, and 4000 Hz; Roverano et al.,12 in a series of 31 patients, identified asymptomatic bilateral SNHL affecting high frequencies. Khalidi et al.9 reported unilateral SNHL involving 500, 1000, 2000, and 3000 Hz associated with a 16% word discrimination score as demonstrated by speech audiometry. Sperling et al.,13 in a series of 84 patients, described bilateral and slowly progressive hearing loss; the same author in a previous paper described patients with SLE who developed sudden unilateral SNHL. Digiovanni and Nair14 and Green and Miller15 presented case reports of sudden hearing loss in patients affected by SLE; in contrast, Andonopoulos et al.17 showed that the threshold of SNHL in patients with SLE mimics the presbycusis pattern. In a large population-based, retrospective cohort study from the Taiwan National Health Insurance Research Database, Lin et al.2 identified a higher prevalence of sudden SNHL in patients with SLE, especially young women, than in the general population; the most common audiological pattern was loss of high-frequency hearing in women aged <35 years. These results were in accordance with the findings by Vendelman et al.,18 who reported bilateral SNHL in patients affected by lupus, hypothesizing a role of vasculitis of the internal auditory artery and stria vascularis. Chawki et al.19 recently presented a case involving a young woman with SLE and bilateral SNHL affecting the 250- to 8000-Hz frequencies. In all studies, the most commonly involved frequencies were in the 4000- to 8000-Hz range (65%), followed by middle frequencies (32%) and low frequencies (3%). The distribution of hearing loss by frequency is shown in Figure 1.
Table 2.
Summary of hearing loss results.
| Authors | Patients (n) | Year | Auditory symptoms | Hearing thresholds |
|---|---|---|---|---|
| Kataoka et al.37 | 2 | 1995 | n/a | Bilateral fluctuant SNHL |
| Sperling et al.13 | 84 | 1998 | 31.0% | Unilateral SNHL (15%), bilateral SNHL (17%) |
| Green and Miller15 | 1 | 2001 | n/a | Sudden pantonal unilateral SNHL, male |
| Digiovanni and Nair14 | 1 | 2006 | n/a | Sudden unilateral SNHL for 3000 and 4000 Hz; male |
| Roverano et al.12 | 31 | 2006 | 70.0% | Bilateral symmetric SNHL for 2000, 4000, and 8000 Hz |
| Gomides et al.19 | 45 | 2007 | 55.5% | SNHL for all frequencies (14%); SNHL for 500, 1000, and 2000 Hz (57%); SNHL for 4000 and 8000 Hz (28%) |
| Khalidi et al.9 | 1 | 2008 | n/a | Profound bilateral SNHL for 500, 1000, 2000, and 3000 Hz; female |
| Maciaszczyk et al.10 | 35 | 2011 | 71.4% | Progressive SNHL with 500-, 2000-, and 4000-Hz frequency preservation |
| Abbasi et al.11 | 45 | 2013 | 26.7% | SNHL (11.1%), otorrhea (8.9%), tinnitus (6.7%) |
| Lin et al.2 | 7168 | 2013 | n/a | Higher incidence of SNHL in women aged ≤34 years. Most frequently affected frequencies: 4000 and 8000 Hz |
| Chawki et al.17 | 1 | 2016 | n/a | Sudden bilateral hearing loss for all frequencies (40 dB in right ear, 60 dB in left ear) |
| Lasso de la Vega et al.16 | 55 | 2016 | 70.0% | High-frequency hearing loss at 8000 and 18,000 Hz |
SNHL, sensorineural hearing loss; n/a, not available
Figure 1.
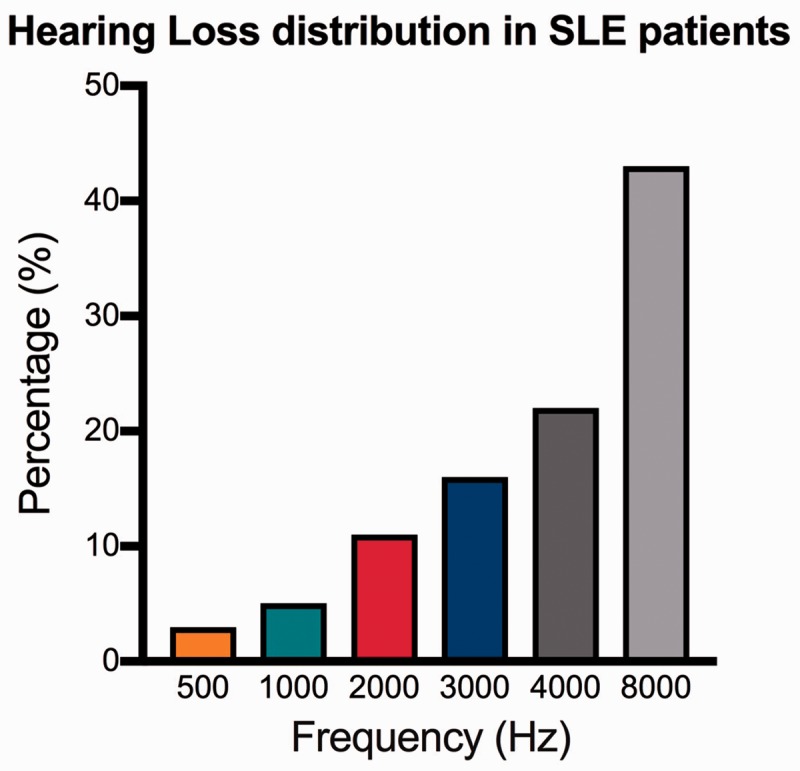
Hearing loss distribution in patients affected by systemic lupus erythematosus.
Besides hearing loss, other audiovestibular symptoms often associated with SLE include tinnitus and vertigo. Tinnitus, a symptom that can be linked to numerous conditions,20–22 has been reported in patients with SLE by Gomides et al.,21 Sperling et al.,13 Dayal and Ellman,20 and Abbasi et al.11 In all cases, tinnitus was associated with hearing loss and may have been a consequence of deafferentation. The vestibular system appears to be involved in SLE, although to a lesser extent; vertigo and dizziness have rarely been reported in patients with lupus. A few authors22,23 have described vertigo in patients with SLE and, in all cases, this symptom was always associated with SNHL or tinnitus. Gad and Abdelateef24 found that vertigo in children with SLE was always associated with cochlear symptoms. However, the incidence of vestibular symptoms may be under-reported due to the slowly progressive onset of these symptoms and compensation by the somatosensory system and vision.
Temporal bone findings
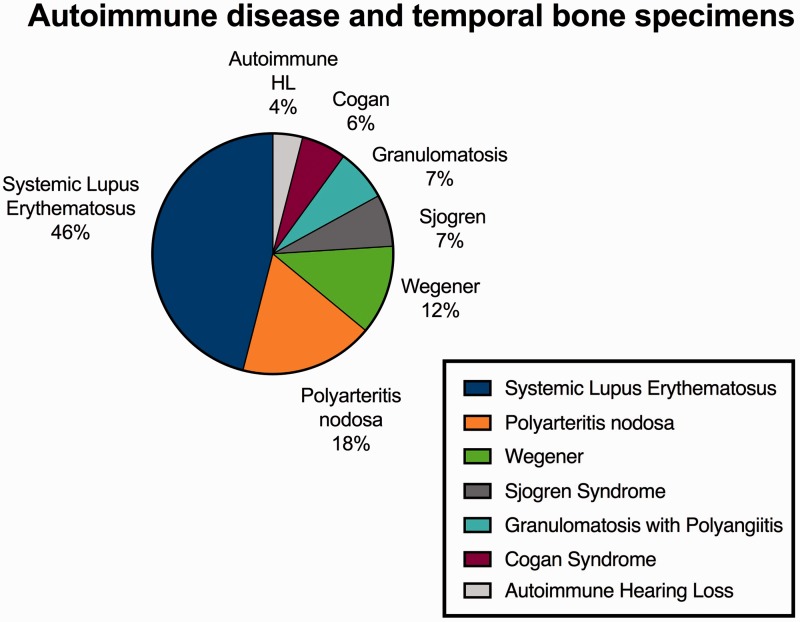
Fourteen publications in which the authors analyzed the effects of different autoimmune diseases on inner ear structures were identified using the following keywords: “auto-immune disease,” “systemic lupus erythematosus (SLE),” “hearing loss,” “temporal bone study,” “vertigo,” “dizziness,” “tinnitus,” “ear symptoms,” “treatment,” “diagnosis,” “symptoms,” “etiopathogenesis,” “Wegener granulomatosis,” “Sjogren,” “polyarteritis nodosa,” “Cogan syndrome,” and “granulomatosis.” Only studies in which the following words were present were included in the review: “SLE,” “temporal bone,” and “hearing symptoms.” Of these studies, 6 analyzed the effect of SLE on the inner ear among a total of 52 analyzed specimens and were selected for the present meta-analysis. The numbers of specimens studied according to autoimmune disease is summarized in Table 3. The different percentages of autoimmune diseases found in the analyzed specimens are shown in Figure 2.
Table 3.
Numbers of specimens studied according to autoimmune disease.
| Autoimmune disease | Specimens studied (n) |
|---|---|
| Systemic lupus erythematosus | 52 |
| Granulomatosis with polyangiitis (Wegener's) | 22 |
| Polyarteritis nodosa | 20 |
| Sjogren syndrome | 8 |
| Cogan syndrome | 7 |
| Autoimmune hearing loss | 4 |
| Total | 113 |
Figure 2.
Autoimmune disease and temporal bone specimens.
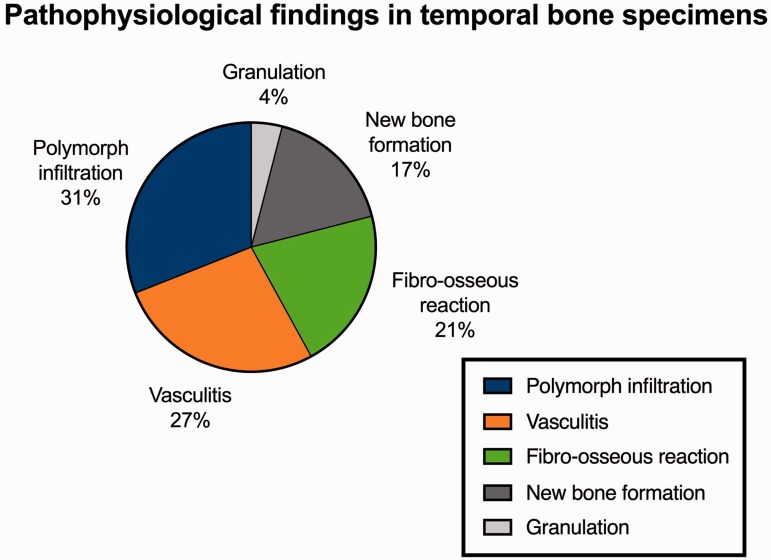
In total, 52 temporal bones, the ones affected by SLE, were included in our meta-analysis. Table 4 summarizes the symptoms that the patients presented before death. The most common condition was hearing loss, found in >70% of patients. Hearing loss was sensorineural in 96% of patients and mixed in 4%. Vertigo and dizziness were reported in 30% of patients. These data are consistent with those reported in the clinical studies described in the previous paragraph. With respect to histopathological findings, polymorphonuclear infiltration (31%) and vasculitis (27%) were the most common, followed by fibro-osseous reaction (21%), new bone formation (17%), and granulation (4%) (Figure 3).
Table 4.
Temporal bone analysis: symptoms reported by patients before death.
| Hearing and vestibular symptoms | Percentage |
|---|---|
| Sensorineural hearing loss | 96% |
| Mixed hearing loss | 4% |
| Vertigo and dizziness | 30% |
| Tinnitus | 8% |
| Aural fullness | 2% |
Figure 3.
Pathophysiological findings in temporal bone specimens.
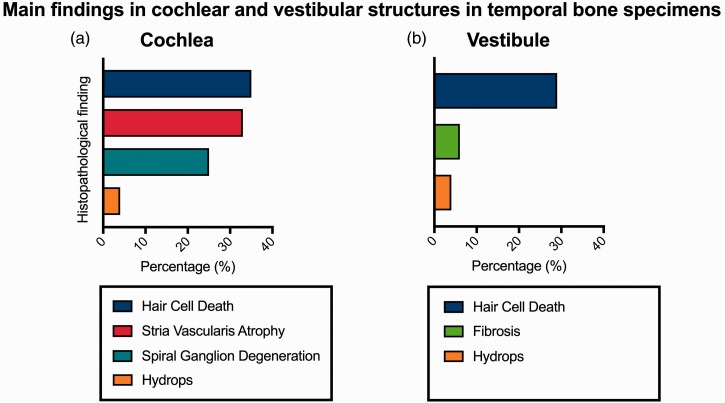
A deeper analysis of cochlear structures showed moderate to severe inner hair cell damage and, mainly, outer hair cell damage in a large portion of studied temporal bones; alterations involved the middle and apical turns of the cochlea in most of the studies,25–28 and all turns were involved in one study.27 Stria vascularis atrophy (33%) and spiral ganglion degeneration (23%) were two other consistent findings. Cochlear hydrops was the least frequently identified histopathological finding (Figure 4a). When focusing on the vestibular system, type I vestibular hair cell damage was the most common finding (29% of cases),28 followed by vestibular fibrosis (6%) and hydrops (4%) (Figure 4b).
Figure 4.
Main findings in cochlear and vestibular structures in temporal bone specimens.
Pathophysiology of inner ear involvement in SLE
SLE can damage tissues and organs through three different mechanisms: 1) antibody/antigen direct reactions, 2) cytotoxic action, and 3) immune complex deposition. These mechanisms represent the basis of inner ear damage in patients with SLE, and a schematic of their connection with specific hearing and vestibular disorders is summarized in Table 5.
Table 5.
Correlations among hearing disorders, temporal bone analysis, and physiopathological mechanisms.
| Hearing and vestibular symptoms | Temporal bone findings | Physiopathological mechanisms |
|---|---|---|
| Hearing loss and tinnitus | Hair cell damage | Anti-DNA antibodies |
| Cytotoxic damage | ||
| Variation of perilymph concentration | ||
| Spiral ganglion Degeneration | Anti-DNA antibodies | |
| Stria vascularis atrophy | Immune complex deposition | |
| Vertigo and dizziness | Type I hair cell damage | Anti-DNA antibodies |
| Cytotoxic damage | ||
| Variation of perilymph concentration |
The autoantibodies produced in patients with SLE are mainly anti-DNA antibodies; their effect changes the DNA conformation, modifying the resultant protein production and inducing apoptosis in cochlear and vestibular hair cells and in the spiral ganglion. In 1988, Barna and Hughes31 identified increased antibody activity in the perilymph of patients with SLE; furthermore, this higher concentration of antibodies increased the protein concentration. These authors also reported that antibodies circulating in the perilymph were associated with the action of cytotoxic molecules and induced a variation in endolymph proteins strictly linked to hair cell degeneration.31
Immune complex deposition plays a central role in the development of inner ear vasculitis and is associated with atrophy of the stria vascularis. Immune complex deposition in the auditory artery reduces the vessel calibre with a consequent decrease in blood flow. This blood flow reduction induces an oxygen deficit followed by the release of oxidative molecules responsible for damage to the hair cells10,11,32,33 and spiral ganglion.34,37 In addition, the progressive reduction of the vessel calibre increases the resistance in the circulatory system, eventually increasing the blood pressure and contributing to damage and fibrosis of the stria vascularis as shown by the temporal bone findings.35
Sudden hearing loss in patients with SLE can be explained by a temporary blood flow reduction in the inner ear, with complete or partial recovery after restoration of normal perfusion. Instead, recurrent vasculitis permanently damages the structure of the ear because of the chronic oxygen deficit.30 Vasculitis appears to be the major factor involved in cochlear and vestibular damage.2,12,13,25,36,37
Green and Miller,15 Karatas et al.,22 Gazquez et al.,38 and Kataoka et al.39 supported the hypothesis that the antibody mechanism can explain cochlear and vestibular hydrops and related hearing/vestibular disorders. However, hydrops seems to be the least common finding in patients with SLE reported in the literature.
Treatment of hearing and vestibular disorders in SLE
Different treatments for SLE-related hearing disorders have been proposed in the literature. These treatments are mainly focused on prevention, especially for slowly progressive hearing loss, and hearing restoration for cases of sudden hearing loss.
Corticosteroid therapy is the most prevalent therapy for sudden hearing loss and for the prevention of further worsening of progressive hearing loss in patients with SLE.11,37,40–43 Sudden hearing loss, as widely reported in the literature, is also subject to spontaneous recovery; Digiovanni and Nair14 reported a case of spontaneous recovery of sudden hearing loss in a patient with SLE.
Other treatments of hearing disorders in patients with SLE include plasmapheresis as reported by Kobayashi et al.44 and Sichkareva et al.,45 anticoagulant therapy,15 and cyclophosphamide.46 These drugs mainly contribute to a reduction in the progression of SNHL.
More recently, the use of monoclonal antibodies such as rituximab or alemtuzumab has been proposed in SLE treatment.47 The suppressive effects of the immune system are able to consistently reduce activation of the inflammatory mechanisms that underlie inner ear damage.
Identification of the pathogenic mechanisms at the basis of audiovestibular symptoms in patients with SLE is crucial for selection of the correct treatment.48–49 Steroids have different activities: anti-inflammatory, immunosuppressive, and anti-oedema effects. Immunosuppression reduces the formation of immune complexes, while anti-inflammatory and anti-oedema actions restore the normal caliber of affected vessels. Additionally, corticosteroid therapy increases blood pressure as a side effect; however, this has a therapeutic effect in patients with SLE because high systemic blood pressure increases blood flow in all structures, including the auditory artery, thus improving the oxygen concentration. Plasmapheresis also improves the oxygen concentration in the inner ear; and anticoagulant therapy improves blood fluidity by increasing vascularization in smaller vessels that have a reduced calibre secondary to SLE. Immunosuppressant drugs such as cyclophosphamide reduce autoimmune activity and the formation of immune complexes.
In the authors' opinion, corticosteroids should be considered as the first treatment option to restore hearing in patients with sudden hearing loss and prevent worsening of progressive hearing loss in patients with SLE because of the large availability of these drugs worldwide and their much lower cost compared with monoclonal antibodies. In patients with progressive hearing loss, cyclophosphamide should be used if steroid therapy is unsuccessful. Plasmapheresis and anticoagulant treatments can be useful for the prevention of sudden hearing loss and treatment of tinnitus and vestibular symptoms; however, their use should be carefully evaluated on an individual basis depending on the patient's systemic involvement and SLE history. Monoclonal antibodies can be a valid choice, especially for aggressive forms of SLE or in patients with proven resistance to other treatments.
Conclusion
The temporal bone studies examined in the present review confirm the aetiopathological mechanisms of SLE in inner ear structures. Two mechanisms are undoubtedly involved at this level: the autoimmune response, supported by the presence of polymorphonuclear leukocytes in the inner ear and the death of hair cells, and the deposition of immune complexes in the vessels, as demonstrated by the presence of vasculitis in the inner ear and by atrophy of the stria vascularis. An understanding of the aetiopathology of SLE through temporal bone findings is certainly helpful in identifying the most effective treatment for SLE in patients with auditory and vestibular disorders. If we assume that temporal bone findings are an exact representation of SLE mechanisms, it appears clear that although corticosteroid therapy is still routinely used for hearing disorders, future research will show that monoclonal antibodies are the gold standard treatment for systemic and specific SLE-related alterations such as inner ear damage.
The use of monoclonal antibodies is often limited to cancer treatment because of their high cost and financial impact on healthcare systems. Although current findings are encouraging, further studies involving larger numbers of patients are necessary to better define the correlations among clinical symptoms, temporal bone findings, and pathological mechanisms in patients with SLE.
Acknowledgments
Special thanks to Dr. Felipe Santos for his review valuable suggestions to Dr. Joseph B. Nadol Jr. for assisting with interpretation of the temporal bone findings.
Declaration of conflicting interest
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Berrih-Aknin S. Myasthenia Gravis: paradox versus paradigm in autoimmunity. J Autoimmun 2014; 52: 1–28. doi: 10.1016/j.jaut.2014.05.001. Epub 2014 Jun 13. Review. [DOI] [PubMed] [Google Scholar]
- 2.Lin C, Lin SW, Weng SF, et al. Risk of sudden sensorineural hearing loss in patients with systemic lupus erythematosus: a population-based cohort study. Audiol Neurootol 2013; 18: 95–100. doi: 10.1159/000345512. Epub 2012 Dec 13. [DOI] [PubMed] [Google Scholar]
- 3.Yamamoto Y, Aoki S. Systemic lupus erythematosus: strategies to improve pregnancy outcomes. Int J Womens Health 2016; 8: 265–272. doi: 10.2147/IJWH.S90157. eCollection 2016. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McCarty DJ, Manzi S, Medsger TA, Jr, et al. Incidence of systemic lupus erythematosus. Race and gender differences. Arthritis Rheum 1995; 38: 1260–1270. [DOI] [PubMed] [Google Scholar]
- 5.Wu M, Yang J, Li X, et al. The role of γδ T cells in systemic lupus erythematosus. J Immunol Res 2016; 2016: 2932531, doi: 10.1155/2016/2932531. Epub 2016 Feb 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cho YN, Kee SJ, Lee SJ, et al. Numerical and functional deficiencies of natural killer T cells in systemic lupus erythematosus: their deficiency related to disease activity. Rheumatology (Oxford) 2011; 50: 1054–1063. doi: 10.1093/rheumatology/keq457. Epub 2011 Jan 27. [DOI] [PubMed] [Google Scholar]
- 7.Chen J, Wu M, Wang J, et al. Immunoregulation of NKT cells in systemic lupus erythematosus. J Immunol Res 2015; 2015: 206731, doi: 10.1155/2015/206731. Epub 2015 Dec 24. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wallace DJ, Hahn BH. Dubois' lupus Erythematosus, 6th ed Philadelphia: Lippincott Williams & Wilkins, 2002. [Google Scholar]
- 9.Khalidi NA, Rebello R, Robertson DD. Sensorineural hearing loss in systemic lupus erythematosus: case report and literature review. J Laryngol Otol 2008; 122: 1371–1376. doi: 10.1017/S0022215108001783. Epub 2008 Feb 19. Review. [DOI] [PubMed] [Google Scholar]
- 10.Maciaszczyk K, Durko T, Waszczykowska E, et al. Auditory function in patients with systemic lupus erythematosus. AurisNasus Larynx 2011; 38: 26–32. doi: 10.1016/j.anl.2010.04.008. Epub 2010 Jun 23. [DOI] [PubMed] [Google Scholar]
- 11.Abbasi M, Yazdi Z, Kazemifar AM, et al. Hearing loss in patients with systemic lupus erythematosus. Glob J Health Sci 2013; 5: 102–106. doi: 10.5539/gjhs.v5n5p102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roverano S, Cassano G, Paira S, et al. Asymptomatic sensorineural hearing loss in patients with systemic lupus erythematosus. J ClinRheumatol 2006; 12: 217–220. [DOI] [PubMed] [Google Scholar]
- 13.Sperling NM, Tehrani K, Liebling A, et al. Aural symptoms and hearing loss in patients with lupus. Otolaryngol Head Neck Surg 1998; 118: 762–765. [DOI] [PubMed] [Google Scholar]
- 14.Digiovanni JJ, Nair P. Spontaneous recovery of sudden sensorineural hearing loss: possible association with autoimmune disorders. J Am AcadAudiol 2006; 17: 498–505. [DOI] [PubMed] [Google Scholar]
- 15.Green L, Miller EB. Sudden sensorineural hearing loss as a first manifestation of systemic lupus erythematosus: association with anticardiolipin antibodies. Clin Rheumatol 2001; 20: 220–222. [DOI] [PubMed] [Google Scholar]
- 16.Lasso de la Vega M, Villarreal IM, López Moya J, et al. Extended high frequency audiometry can diagnose sub-clinic involvement in a seemingly normal hearing systemic lupus erythematosus population. Acta Otolaryngol 2016, pp. 1–6. [DOI] [PubMed] [Google Scholar]
- 17.Andonopoulos AP, Naxakis S, Goumas P, et al. Sensorineural hearing disorders in systemic lupus erythematosus. A controlled study. Clin Exp Rheumatol 1995; 13: 137–141. [PubMed] [Google Scholar]
- 18.Vendelman JE, Roord JJ, O’Connor AF, et al. Autoimmunity and ear disorders: a immune-complex mediated sensorineurl hearing loss. Laryngoscope 1984; 94: 501–507. [DOI] [PubMed] [Google Scholar]
- 19.Chawki S, Aouizerate J, Trad S, et al. Bilateral sudden sensorineural hearing loss as a presenting feature of systemic lupus erythematosus: Case report and brief review of other published cases. Medicine (Baltimore) 2016; 95: e4345–e4345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dayal VS, Ellman MH. Sensorineural hearing loss and lupus. J Rheumatol 1999; 26: 2065–2065. [PubMed] [Google Scholar]
- 21.Gomides AP, Do Rosário EJ, Borges HM, et al. Sensorineural dysacusis in patients with systemic lupus erythematosus. Lupus 2007; 16: 987–990. [DOI] [PubMed] [Google Scholar]
- 22.Karatas E, Onat AM, Durucu C, et al. Audiovestibular disturbance in patients with systemic lupus erythematosus. Otolaryngol Head Neck Surg 2007; 136: 82–86. [DOI] [PubMed] [Google Scholar]
- 23.Batuecas-Caletrío A, del Pino-Montes J, Cordero-Civantos C, et al. Hearing and vestibular disorders in patients with systemic lupus erythematosus. Lupus 2013; 22: 437–442. [DOI] [PubMed] [Google Scholar]
- 24.Gad GI, Abdelateef H. Function of the audiovestibular system in children with sys-temic lupus erythematosus. Curr Allergy Asthma Rep 2014; 14: 446–446. [DOI] [PubMed] [Google Scholar]
- 25.Kariya S, Kaya S, Hizli O, et al. Cochlear Histopathologic Findings in Patients With Systemic Lupus Erythematosus: A Human Temporal Bone Study. Otol Neurotol 2016; 37: 593–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yoon TH, Paparella MM, Schachern PA. Systemic vasculitis: a temporal bone histopathologic study. Laryngoscope 1989; 99(6 Pt 1): 600–609. [DOI] [PubMed] [Google Scholar]
- 27.Sone M, Schachern PA, Paparella MM, et al. Study of systemic lupus erythematosus in temporal bones. Ann Otol Rhinol Laryngol 1999; 108: 338–344. [DOI] [PubMed] [Google Scholar]
- 28.Fukushima N, Fukushima H, Cureoglu S, et al. Hearing loss associated with systemic lupus erythematosus: temporal bone histo-pathology. Otol Neurotol 2006; 27: 127–128. [DOI] [PubMed] [Google Scholar]
- 29.Hoistad DL, Schachern PA, Paparella MM. Autoimmune sensorineural hearing loss: a human temporal bone study. Am J Otolaryngol 1998; 19: 33–39. [DOI] [PubMed] [Google Scholar]
- 30.Kariya S, Hızlı O, Kaya S, et al. Histopathologic findings in peripheral ves-tibular system from patients with systemic lupus erythematosus: a human temporal bone study. Otol Neurotol 2015; 36: 1702–1707. [DOI] [PubMed] [Google Scholar]
- 31.Barna BP, Hughes GB. Autoimmunity and otologic disease: clinical and experimental aspects. Clin Lab Med 1988; 8: 385–398. [PubMed] [Google Scholar]
- 32.Eckhard A, Gleiser C, Arnold H, et al. Water channel proteins in the inner ear and their link to hearing impairment and deafness. Mol Aspects Med 2012; 33: 612–637. [DOI] [PubMed] [Google Scholar]
- 33.Olivetto E, Simoni E, Guaran V, et al. Sensorineural hearing loss and ischemic injury: development of animal models to assess vascular and oxidative effects. Hear Res 2015; 327: 58–68. [DOI] [PubMed] [Google Scholar]
- 34.Calabrese V, Cornelius C, Maiolino L, et al. Oxidative stress, redox homeostasis and cellular stress response in Meniere’s disease: role of vitagenes. Neurochem Res 2010; 35: 2208–2217. doi: 10.1007/s11064-010-0304-2. [DOI] [PubMed] [Google Scholar]
- 35.Johnsson LG. Vascular pathology in the human inner ear. Adv Otorhinolaryngol 1973; 20: 197–220. [DOI] [PubMed] [Google Scholar]
- 36.Kurata N, Schachern PA, Paparella MM, et al. Histopathologic Evaluation of Vascular Findings in the Cochlea in Patients With Presbycusis. JAMA Otolaryngol Head Neck Surg 2016; 142: 173–178. [DOI] [PubMed] [Google Scholar]
- 37.Bowman CA, Linthicum FH, Jr, Nelson RA, et al. Sensorineural hearing loss associated with systemic lupus erythematosus. Otolaryngol Head Neck Surg 1986; 94: 197–204. [DOI] [PubMed] [Google Scholar]
- 38.Gazquez I, Soto-Varela A, Aran I, et al. High prevalence of systemic autoimmune diseases in patients with Menie`re’s disease. PLoS One 2011; 6: e26759–e26759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kataoka H, Takeda T, Nakatani H, et al. Sensorineural hearing loss of suspected autoimmune etiology: a report of three cases. AurisNasus Larynx 1995; 22: 53–58. [DOI] [PubMed] [Google Scholar]
- 40.Caldarelli DD, Rejowski JE, Corey JP. Sensorineural hearing loss in lupus erythe-matosus. Am J Otol 1986; 7: 210–213. [PubMed] [Google Scholar]
- 41.Cordeschi S, Salvinelli F, D’Ascanio L. Sensorineural hearing impairment in systemic lupus erythematosus: sudden or progressive? Clin Exp Rheumatol 2004; 22: 653–653. [PubMed] [Google Scholar]
- 42.Kobayashi S, Fujishiro N, Sugiyama K. Systemic lupus erythematosus with sensori-neural hearing loss and improvement after plasmapheresis using the double filtration method. Intern Med 1992; 31: 778–781. [DOI] [PubMed] [Google Scholar]
- 43.Sichkareva TA, Vishniakov VV, Kutepov DE. [The role of plasmapheresis in the treatment of patients with sensorineural deafness]. Vestn Otorinolaringol 2009; 3: 36–38. [in Russian, English Abstract]. [PubMed] [Google Scholar]
- 44.McCabe BF. Autoimmune inner ear disease: results of therapy. Adv Otorhinolaryngol 1991; 46: 78–81. [DOI] [PubMed] [Google Scholar]
- 45.Dasgupta S. Therapeutic interventions of tissue specific autoimmune onset in systemic lupus erythematosus. Mini Rev Med Chem 2016. [DOI] [PubMed]
- 46.Petri M. Systemic lupus erythematosus and related diseases: clinical features. In: Rose NR, Mackay IR. (eds). The autoimmune dis-eases, 4th ed St Louis: Elsevier Academic Press, 2006, pp. 351–356. [Google Scholar]
- 47.Gussen R. Sudden deafness of vascular origin: a human temporal bone study. Ann Otol Rhinol Laryngol 1976; 85(1 Pt 1): 94–100. [DOI] [PubMed] [Google Scholar]
- 48.Mok CC, Lau CS. Pathogenesis of systemic lupus erythematosus. J ClinPathol 2003; 56: 481–490. [DOI] [PMC free article] [PubMed] [Google Scholar]