Abstract
Objectives
In the general population, metabolic health (MH) often declines as body mass index (BMI) increases. However, some obese individuals maintain MH. HIV and antiretroviral therapy (ART) have been associated with metabolic disturbances. We hypothesized that HIV-infected (HIV+) men on suppressive ART experience less MH than HIV-uninfected (HIV−) men across all BMI categories.
Design/Methods
In a cross-sectional analysis of 1018 HIV+ and 1092 HIV− men enrolled in the Multicenter AIDS Cohort Study, Poisson regression with robust variance determined associations between HIV serostatus and MH prevalence (defined as meeting ≤2 of 5 NCEP/ATP III metabolic syndrome criteria), adjusting for age, race, BMI category, smoking and hepatitis C virus infection status.
Results
HIV+ men were younger (54 vs 59 years) and had lower median BMI (25 vs 27 kg/m2). Non-obese HIV+ men had lower MH prevalence than HIV− men (BMI ≤25 kg/m2: 80% vs 94%, p<0.001; BMI 25–29 kg/m2: 64% vs 71%, p=0.05), but MH prevalence among obese men did not differ by HIV serostatus (BMI 30–34 kg/m2: 35% vs 39%, p=0.48; BMI ≥35 kg/m2: 27% vs 25%, p=0.79). In the adjusted model, non-obese HIV+ men were less likely to demonstrate MH than non-obese HIV− men. Among HIV+ men, per year darunavir, zidovudine and stavudine use were associated with lower MH likelihood.
Conclusions
Metabolically healthy obesity prevalence does not differ by HIV serostatus. However, among non-obese men, HIV infection is associated with lower MH prevalence, with associations between lack of MH and darunavir and thymidine analog nucleoside reverse transcriptase inhibitor exposure observed.
Keywords: HIV, obesity, metabolic syndrome, metabolically healthy obesity, thymidine analog, darunavir
Introduction
Overweight and obese states are common among HIV-infected persons,[1, 2] with some cohorts reporting the frequency of body mass index (BMI) ≥25 kg/m2 among HIV-infected adults to be 44%–65%.[1, 3] Similar to the general population, obesity in HIV infection is associated with multi-morbidity.[1, 4–6] Even in normal weight persons, HIV infection and antiretroviral therapy (ART) are associated with metabolic derangements including fat redistribution, adipocyte dysfunction, increased cardiovascular disease risk, insulin resistance and dyslipidemia.[7] Problematically, ART may exacerbate obesity: In an observational cohort, 44% of HIV-infected adults were overweight or obese prior to ART initiation, and 20% progressed from normal or overweight to overweight or obese within two years of ART initiation.[1] Since HIV infection and obesity are both chronic inflammatory states,[8, 9] their co-existence may hasten metabolic dysfunction. Thus, obesity is often assumed to be a deleterious state that exacerbates HIV− and ART-associated metabolic issues.
In the general population, a state of metabolically healthy obesity (MHO) has been described. MHO is obesity without overt concomitant cardiometabolic disease, and has been variably defined to include persons with BMI ≥25 or ≥30 kg/m2 and most commonly ≤2 metabolic syndrome criteria.[10–13] The lack of consistent diagnostic criteria and population differences has led to MHO prevalence estimates ranging from 6–40%;[14] furthermore, whether MHO exists at all is controversial. However, some studies have demonstrated increased mortality, increased cardiovascular disease risk and differences in body composition and fat distribution among persons meeting MHO criteria in the general population compared to metabolically healthy, non-obese persons,[14–21] suggesting that MHO is not entirely benign. In contrast, persons with MHO may have less visceral fat and systemic inflammation and more favorable immune profiles than the metabolically unhealthy obese,[22–26] suggesting that a spectrum of metabolic health can co-exist within obesity.
It is unknown whether MHO exists among HIV-infected persons. Given that HIV infection and ART are associated with a variety of metabolic changes, that the prevalence of the metabolic syndrome in HIV-infected persons is high (up to 45% vs 25% in the general population[27]) and that immunologic dysregulation and enhanced inflammation may exist independent of obesity in treated and untreated HIV-infected persons, it is possible that HIV infection and obesity have a synergistic and detrimental effect on metabolic health that could prevent the existence of MHO. We used Multicenter AIDS Cohort Study (MACS) data to: determine the prevalence of MHO among HIV-infected MACS men, compare metabolic health by BMI category and age between HIV-infected and HIV-uninfected men and compare predictors of metabolic health by HIV serostatus. We hypothesized that HIV-infected men would be less metabolically healthy across the BMI spectrum and would have a lower prevalence of MHO.
Methods
Study population
The MACS began in 1984 to study the natural history of AIDS among men who have sex with men, to identify risk factors for the occurrence and clinical expression of HIV infection and to establish a repository of biologic specimens for future study.[28] The MACS is an ongoing, multicenter (Pittsburgh, PA; Baltimore, MD/Washington, DC; Chicago, IL; and Los Angeles, CA), prospective, observational cohort study in which participants return semi-annually for a standardized interview, clinical evaluations, laboratory tests and specimen storage. Beginning April 1, 2009 (Visit 31), fasting evaluations for metabolic data became routinely available. We conducted a nested cohort study of MACS participants who were alive at Visit 31 or later, using data from the most recent study visit for each participant after April 1, 2009 but before September 30, 2014 to assess metabolic health status. In addition, we restricted HIV-infected men to those receiving ART and with suppressed plasma HIV-1 RNA (<50 copies/mL by Ultrasensitive Amplicor HIV-1 Monitor Assay, Roche Diagnostics, Pleasanton, CA) at the visit of interest. All participants provided informed consent, protocols were approved by the Institutional Review Boards of the participating sites and the study was conducted in accordance with the Declaration of Helsinki.
HIV Diagnosis
Peripheral venous blood is screened semi-annually for HIV-uninfected men using ELISA and positive specimens are confirmed using western blot. After HIV-infection is confirmed, semiannual testing is discontinued (for HIV-infected men).
Metabolic definitions
We defined metabolic health using the National Cholesterol Education Program Adult Treatment Panel III (NCEP ATP III) definition, which requires the presence of ≤2 of the following: Systolic blood pressure (sbp) >130 mmHg, diastolic blood pressure (dbp) >85 mmHg, triglycerides >150 mg/dL, high-density lipoprotein cholesterol (HDL) <40 mg/dL, fasting plasma glucose >100 mg/dL and waist circumference >102 cm.[10, 11] Use of blood pressure-, lipid- or glucose-lowering medications qualified a participant as meeting the respective criterion. BMI categories were defined as <25.0, 25.0–29.9, 30.0–34.9 and ≥35.0 kg/m2. We additionally created 4 categories combining metabolic status and BMI: MHO (BMI ≥30 kg/m2 with metabolic health), metabolically unhealthy obese (BMI ≥30 kg/m2 and >2 of the metabolic criteria), metabolically healthy non-obese (BMI <30 kg/m2 with metabolic health) and metabolically unhealthy non-obese (BMI <30 kg/m2 and >2 of the metabolic criteria).
Other Measurements
Age, race, level of education, smoking history, medication use and diagnosis history were assessed by self-report, with current metabolic diagnoses confirmed by laboratory or medication use. AIDS and other clinical events were confirmed via medical record review. Depression was defined as Center for Epidemiologic Studies Depression Scale Score >16 or diagnosis of or treatment for depression. Chronic hepatitis C virus (HCV) infection was defined as plasma HCV RNA positivity. Height and weight were measured using standardized procedures and used to calculate the BMI in kg/m2. Waist circumference (cm) at the iliac crest was measured using a standardized protocol.[29] All socio-demographic and medical data were obtained using the most recent visit with complete metabolic data up to September 30, 2014. CD4+ T lymphocyte subsets were measured using standardized flow cytometry,[30] and CD4+ T lymphocyte nadir was defined as the lowest count prior to and including the most recent visit with complete metabolic data.
Statistical Analysis
A descriptive analysis of demographic and clinical characteristics was generated and stratified by HIV serostatus. Continuous variables are expressed as mean ± standard deviation if normally distributed or median with interquartile range if not normally distributed. Wilcoxon rank-sum tests and Pearson χ2 tests compared continuous and categorical characteristics, respectively. All statistical tests are two-sided (α=0.05). No adjustment was made for multiple testing in these exploratory analyses.
The prevalence of metabolic health initially assessed by HIV serostatus, BMI category and type of metabolic disturbance was explored over both the 5 years of data from April 1, 2009 to September 30, 2014 and the last visit with complete metabolic data, which for most men was their last MACS visit within the study period. Results and missing data frequency (approximately 5%) were similar between the 2 analyses with high intra-class correlation coefficients for metabolic health status (HIV-uninfected 0.86, 95% CI 0.82, 0.86; HIV-infected 0.79, 95% CI 0.77, 0.82; data not shown), and participants rarely changed metabolic health status over the 5 years. Therefore, metabolic health at the last visit was chosen for subsequent analysis.
Missing metabolic health data was imputed using an individual participant’s metabolic health data from 1 year before to 2.5 years after the current visit (but closest to the current/missing visit). Individual trajectories were chosen over group medians as, compared to the men with complete data (n=2380), men with missing data (n=329) were more likely to have diabetes mellitus (38% vs 14%, p<0.001) and dyslipidemia (88% vs 75%, p<0.001), had longer time with known HIV infection (22 years vs 12 years, p<0.001) and were less likely to have HIV-1 RNA <50 copies/mL (66% vs 82%, p=0.001). Wilcoxon rank sum tests compared differences in type of metabolic disturbance and the distributions of metabolic categories, both by HIV serostatus.
Poisson regression with robust variance determined factors associated with metabolic health. Separate models were performed for cohort enrollment status (before or after 2001), education (≤high school vs. >high school), current smoking status and HCV infection, controlling for age, race (white vs. non-white), HIV serostatus, BMI category and the interaction of HIV infection with BMI, to determine covariates potentially associated with metabolic health status. Final multivariable Poisson regression models determined associations between HIV serostatus and metabolic health prevalence, adjusting for variables significant (p<0.05) in the previous analysis (age, race, current smoking and HCV status). Next, HIV-restricted models were developed to assess the role of HIV- and ART-specific covariates and included ART duration, current CD4+ T lymphocyte count, nadir CD4+ T lymphocyte count, history of an AIDS diagnosis and/or a diagnosis of lipodystrophy (in addition to age, race, education, current smoking and HCV status). Interactions between covariates were explored but not observed unless specified. Interpretations of associations were made giving special consideration to the possibility that specific covariates may also be in the causal pathway for dysmetabolism. Multiple imputation was used to complete missing covariate data (5%). Five imputation data sets were created using multivariable normal model, including all variables in the Poisson model. The final estimates of the association between metabolic health and factors examined were obtained by averaging the estimates from the five imputation data sets.
Results
Participant population
Demographic and clinical characteristics are presented in Table 1. Of 1092 HIV-uninfected and 1018 HIV-infected participants on ART with HIV RNA<50 copies/mL, HIV-infected men were younger, more likely to be of non-white race, have ≤high school education and to be current smokers. HIV-infected men had slightly lower BMIs and median waist circumference, and were more likely to have dyslipidemia and HCV infection, but had similar rates of diabetes mellitus and metabolic syndrome as HIV-uninfected men.
Table 1.
Clinical and Demographic Factors
| Characteristic | HIV-infected (n) | HIV-infected* | HIV-uninfected (n) | HIV-uninfected* | P value |
|---|---|---|---|---|---|
| Age (years) | 1018 | 53.7 (46.0, 60.4) | 1092 | 58.9 (51.7, 65.6) | <0.001 |
| Non-white race | 1018 | 43% | 1092 | 29% | <0.001 |
| ≤High school education | 1015 | 22% | 1091 | 15% | <0.001 |
| Pre-2001 enrollment cohort | 1018 | 46% | 1092 | 66% | <0.001 |
| Current smoking | 991 | 26% | 1042 | 19% | <0.001 |
| BMI (kg/m2) | 1018 | 25.1 (22.8, 28.4) | 1092 | 26.8 (23.8, 30.1) | <0.001 |
| Waist circumference (cm) | 929 | 93.9 (86.5, 102.6) | 988 | 98.4 (89.3, 108.5) | <0.001 |
| Hypertension | 981 | 48% | 1047 | 52% | 0.06 |
| Diabetes mellitus | 851 | 15% | 942 | 13% | 0.14 |
| Metabolic syndrome | 1018 | 33% | 1092 | 30% | 0.14 |
| Dyslipidemia | 953 | 77% | 1034 | 73% | 0.02 |
| HCV infection | 986 | 7% | 1087 | 5% | 0.03 |
| Depression | 927 | 25% | 991 | 22% | 0.15 |
| Years since HIV diagnosis | 1018 | 12.2 (9.2, 27.2) | |||
| Current CD4+ T cell count (cells/μL) | 1016 | 655 (481, 855) | |||
| Nadir CD4+ T cell count (cells/μL) | 1018 | 262 (151, 371) | |||
| % visits with HIV-1 RNA <50 copies/mL | 1018 | 89 ± 19 | |||
| AIDS diagnosis | 1018 | 13% | |||
| Current PI use** | 1018 | 42% | |||
| Cumulative PI use (years) | 709 | 7.6 (3.5, 12.4) | |||
| Current NNRTI use | 1018 | 51% | |||
| Cumulative NNRTI use (years) | 810 | 5.9 (2.2, 10.9) | |||
| Current NRTI use*** | 1018 | 92% | |||
| Cumulative NRTI use (years) | 1017 | 12.4 (5.6, 16.8) | |||
| Current INSTI use | 1018 | 29% | |||
| Cumulative INSTI use (years) | 320 | 2.2 (0.9, 4.6) |
Current darunavir use=18.4%
Current zidovudine use=6.5%; current stavudine use=0.5%
IQR= interquartile range; BMI=body mass index; HCV=hepatitis C virus; PI=protease inhibitor; NNRTI=non-nucleoside reverse transcriptase inhibitor; NRTI=nucleoside reverse transcriptase inhibitor; INSTI=integrase inhibitor
Among the HIV-infected men, median time since HIV diagnosis was 12.2 years, with median current CD4+ T lymphocyte count 655 cells/μL. In addition to virologic suppression at the visit associated with metabolic health determination, participants overall exhibited durable suppression, with HIV-1 RNA <50 copies/mL at nearly 90% of visits in the 5 years preceding the visit of metabolic health determination. Thirteen percent had a diagnosis of AIDS. Current ART use included 42% protease inhibitor (PI) use, 51% non-nucleoside reverse transcriptase inhibitor (NNRTI) use and 29% integrase inhibitor use, with 92% of men being on ≥1 NRTI.
Components of the Metabolic Health Definition
HIV-infected men were more likely to meet elevated triglyceride and low HDL cholesterol criteria, whereas HIV-uninfected men were more likely to meet waist circumference criteria (Tables 1 and 2). Elevated blood pressure and blood glucose frequencies were similar by HIV serostatus. Frequency of all metabolic syndrome components increased with increasing BMI, with patterns not varying by HIV serostatus by the time BMI exceeded 35 kg/m2.
Table 2.
Metabolic Disturbances by HIV Serostatus and BMI Category
| Elevated blood pressure* | Elevated triglycerides* | Low HDL cholesterol | Elevated blood glucose* | Elevated waist circumference | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HIV+ | HIV− | HIV+ | HIV− | HIV+ | HIV− | HIV+ | HIV− | HIV+ | HIV− | |
| BMI <25 kg/m2 | 53% | 50% | 47% | 33% | 25% | 7% | 28% | 21% | 3% | 2% |
| BMI 25–29 kg/m2 | 62% | 68% | 53% | 45% | 35% | 22% | 34% | 29% | 26% | 36% |
| BMI 30–34 kg/m2 | 81% | 70% | 63% | 62% | 47% | 34% | 49% | 49% | 80% | 79% |
| BMI ≥35 kg/m2 | 78% | 83% | 70% | 59% | 44% | 49% | 53% | 56% | 88% | 85% |
| Overall | 60% | 63% | 52% | 45% | 32% | 21% | 34% | 32% | 24% | 35% |
Diagnosis or receiving medical therapy
HDL=high-density lipoprotein cholesterol, HIV=human immunodeficiency virus, BMI=body mass index
Numbers in bold font indicate p-value <0.05 comparing HIV+ and HIV− within each BMI category and each metabolic component.
Metabolic Health by BMI and HIV Serostatus
Unadjusted frequencies of metabolic health by BMI and HIV serostatus are presented in Figure 1. Overall, 72% of HIV-uninfected and 67% of HIV-infected men met criteria for metabolic health. However, normal weight (BMI<25.0 kg/m2) and overweight (BMI=25.0–29.9 kg/m2) HIV-infected men were less likely to be metabolically healthy than HIV-uninfected men in the same BMI categories. However, once BMI exceeded 30 kg/m2, rates of metabolic health did not differ by HIV serostatus, confirming the presence of metabolically healthy obesity among HIV-infected men.
Figure 1.
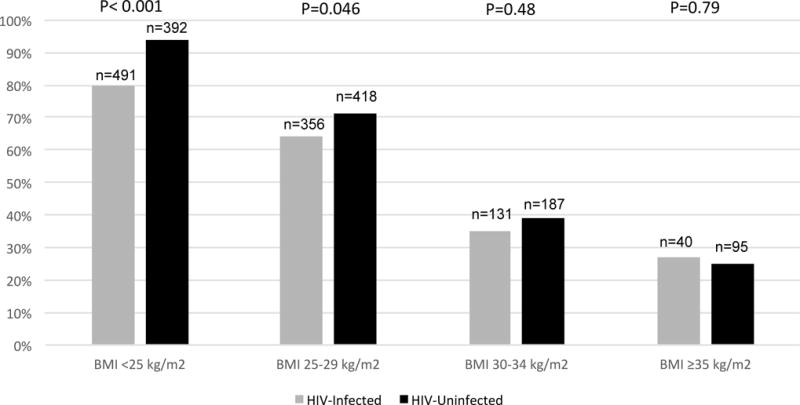
Percent Metabolic Health by BMI Category and HIV Serostatus
After adjusting for age, race, current smoking and HCV infection status, HIV-infected normal weight and overweight men remained significantly less likely to experience metabolic health compared to HIV-uninfected men in the same BMI category (Table 3). Similar to the unadjusted analyses, the prevalence of metabolic health did not differ by HIV serostatus at BMI>30 kg/m2.
Table 3.
Effect of HIV Infection on Metabolic Health by BMI Category
| Adjusted Prevalence Ratio (95% CI) for Metabolic Health in HIV-Infected vs HIV-Uninfected* | P value | |
| BMI <25 kg/m2 (n=883) | 0.81 (0.77, 0.85) | <0.001 |
| BMI 25–29 kg/m2 (n=774) | 0.84 (0.77, 0.93) | <0.001 |
| BMI 30–34 kg/m2 (n=318) | 0.85 (0.64, 1.14) | 0.27 |
| BMI ≥35 kg/m2 (n=135) | 1.05 (0.58, 1.92) | 0.86 |
Adjusted for age, race, current smoking and hepatitis C virus infection status.
HIV=human immunodeficiency virus, BMI=body mass index, CI=confidence interval
Factors Associated with Metabolic Health Status Among HIV-infected Men
In an HIV-restricted analysis, factors significantly associated with decreased likelihood of metabolic health included higher BMI, every additional ten years of age, white race, and per year PI, zidovudine and stavudine use (Table 4). Current smoking, HCV infection, history of an AIDS diagnosis and nadir CD4+ T lymphocyte count were not associated with metabolic health status.
Table 4.
Factors Associated with Metabolic Health Among HIV-Infected Men
| Adjusted Prevalence Ratio (PR) for Metabolic Health | ||
|---|---|---|
| PR (95% CI) | P value | |
| BMI 25–29 kg/m2* | 0.78 (0.72, 0.85) | <0.001 |
| BMI 30–34 kg/m2* | 0.42 (0.33, 0.53) | <0.001 |
| BMI ≥35 kg/m2 * | 0.33 (0.20, 0.54) | <0.001 |
| Age per 10 years | 0.96 (0.92, 0.99) | 0.02 |
| White race§ | 0.88 (0.81, 0.96) | 0.003 |
| Current Smoking⌘ | 0.94 (0.86, 1.02) | 0.13 |
| HCV co-infection | 0.87 (0.72, 1.05) | 0.15 |
| Current CD4+ T lymphocyte count <500 cells/μL | 1.09 (1.00, 1.18) | 0.05 |
| Per year PI use | 0.99 (0.98, 1.00) | 0.005 |
| Per year zidovudine use | 0.98 (0.97, 0.99) | <0.001 |
| Per year stavudine use | 0.98 (0.96, 1.00) | 0.02 |
Reference BMI<25 kg/m2
Reference non-white race
Reference current non-smokers
HIV=human immunodeficiency virus, BMI=body mass index, HCV=hepatitis C virus infection, PI=protease inhibitor
Given the finding of decreased metabolic health likelihood per year of PI use, we conducted an analysis restricted to men with PI exposure (n=709). First, we created models adjusting for the factors significantly associated with metabolic health in the parent model (BMI, age, race, current smoking, HCV infection, CD4+ T lymphocyte count<500 cells/μL), then added individual PIs to the model, including ritonavir (boosting or full dose). Only darunavir use was significantly associated with lack of metabolic health, with each year of use conferring a 5% decreased likelihood of metabolic health (Table S1a). Next, we added zidovudine, stavudine and ritonavir use to the parent model (to account for other agents associated with decreased risk of metabolic health and concomitant ritonavir use with darunavir) and again assessed each PI individually. Again, darunavir use was the only PI associated with frequency of metabolic health (effect size unchanged, Table S1b).
Of note, compared to other PI users (n=490), men who had ever used darunavir (n=219): were slightly younger (53 vs 56 years, p<0.001); were more likely to be non-white (48% vs 36%, p=0.003); had shorter known duration of HIV infection (12 vs 22 years, p<0.001) but lower current CD4+ T lymphocyte counts (current: 583 vs 646 cells/μL, p=0.008; trend towards lower nadir CD4+ T lymphocyte count also observed, 209 vs 239 cells/μL, p=0.09) and less durable virologic suppression (83% vs 91% of visits with HIV-1 RNA<50 copies/mL, p<0.001); were less likely to currently use NRTIs (78% vs 94%, p<0.001) but more likely to have previously used stavudine (but not zidovudine, 51% vs 43%, p=0.04); and were more likely to currently be receiving an integrase inhibitor (42% vs 26%, p<0.001).
A statistically significant interaction between darunavir and zidovudine (prevalence ratio [PR]=0.98, p=0.004) but not darunavir and stavudine use (PR=0.99, p=0.26) was observed. Specifically, we observed that cumulative darunavir use had no effect on metabolic health when cumulative zidovudine use was <3 years, but darunavir use was negatively associated with metabolic health when cumulative zidovudine use was ≥3 years. For example, the estimate for darunavir use when zidovudine use is 3 years in duration was PR=0.96, (95% CI 0.93, 0.996), p=0.03. At 4 years of zidovudine use the estimate for darunavir was PR= 0.94, (95% CI 0.91, 0.98), p=0.006. Additionally, darunavir users did not differ in their metabolic profiles from other PI users, nor did they differ in frequency of current smoking or HCV co-infection (data not shown). Of the components of the metabolic health definition, DRV use was statistically associated with elevated triglyceride (adjusted PR 1.05; 95% CI 1.03, 1.07; p<0.001) and low HDL (adjusted PR 1.07; 95%CI 1.02, 1.12; p=0.01) cholesterol levels.
Discussion
From the MACS, a large, prospective cohort of HIV-infected and HIV-uninfected men, we present the first analysis documenting the presence of MHO in HIV-infected persons. We also observed that MHO exists among HIV-infected men on suppressive ART to a similar extent as among HIV-uninfected men. Our third and equally important finding was that non-obese HIV-infected men were significantly less likely than HIV-uninfected men in the same BMI category to be metabolically healthy. And, while BMI had the greatest impact on metabolic health status, older age, white race and ART use, specifically per year PI (darunavir), zidovudine and stavudine use, were also associated with reduced likelihood of metabolic health among HIV-infected men.
MHO prevalence has not previously been assessed in HIV-infected persons, and the finding of similar MHO frequencies between HIV-infected and HIV-uninfected men was 1) unexpected and 2) provides evidence against the hypothesis that chronic HIV infection and obesity have synergistic effects on metabolic dysregulation. Given the numerous metabolic alterations associated with HIV infection and long-term ART use (including prominent lipid abnormalities and high rates of insulin resistance[31, 32] and metabolic syndrome[27]), we hypothesized that HIV-infected men on suppressive ART would be less likely to have MHO than HIV-uninfected controls. Importantly, rates of MHO in our cohort are similar to those previously reported in the general population: At BMI 30–34 kg/m2 we observed MHO rates of 35% and 39% for HIV-infected and HIV-uninfected men, respectively, and 27% and 25% maintained MHO even at BMI ≥35 kg/m2. Using the same definition of MHO (≤2 NCEP ATPIII criteria), the prevalence of MHO in the U.S. general population is 29.0–31.7%.[12, 33] Thus, our data in HIV-uninfected men are consistent with that of the general population, lending validity to the estimate for ART-treated, HIV-infected men.
Among normal weight men in our cohort, HIV-infected men were less likely than HIV-uninfected men to be metabolically healthy, although non-obese HIV-infected men still retained greater rates of metabolic health than obese men of any HIV serostatus. In other U.S. studies, approximately 30% of normal weight, HIV-uninfected persons lack metabolic health.[33] Risk factors for lack of metabolic health among normal weight adults are less well characterized, but may include older age and substance use.[34] Among MACS men with BMI<25 kg/m2, older age, white race, HIV infection and HCV infection were most strongly associated with a lack of metabolic health (data not shown). For HIV-infected men, per year PI and thymidine analog NRTI use were also associated with lack of metabolic health.
Given toxicities including lipodystrophy, mitochondrial dysfunction, hepatic steatosis and lactic acidosis,[35–37] it is not surprising that zidovudine and stavudine use were associated with lack of metabolic health. The magnitude of the association, however, was impressive: At 2% per year, 10 years of any combination of zidovudine or stavudine use would decrease the risk of maintaining metabolic health by 20%. Given that ART use is currently a lifetime commitment and these agents are still frequently used in resource-limited settings, the potential contribution of ART to metabolic disease in persons on long-term therapy is substantial. Additionally, our analysis was not limited to current zidovudine and stavudine users, highlighting the potential long-lasting effects of these agents.
Similarly, PI use has been associated with metabolic disturbances including lipodystrophy, insulin resistance and dyslipidemia,[37] and we were not surprised to observe an association between decreased frequency of metabolic health and PI exposure. However, it was surprising to observe a specific association with darunavir use. Historically, darunavir has been thought of as more metabolically neutral than many other PIs, with the possible exception of atazanavir.[38–40] However, in our cohort darunavir use was initially more likely to have been initiated in the face of treatment experience, in which twice daily dosing is more common. Only after 2011 did the frequency of once daily darunavir dosing exceed 50% among MACS men.
Twice daily darunavir has been associated with greater lipid perturbations than once daily dosing,[41] as well as greater ritonavir exposure. Our association between darunavir use and decreased frequency of metabolic health did persist after controlling for ritonavir usage. However, men in our cohort may have been switched to darunavir after developing metabolic disease on other ART agents, and that we were not fully able to account for such overlapping processes in this analysis. This hypothesis is supported by our finding of an interaction between darunavir and zidovudine use, such that darunavir use was only statistically significantly associated with reduced likelihood of metabolic health when zidovudine use occurred for at least 3 years, highlighting the confounding effects of treatment experience.
Of note, participants in AIDS Clinical Trials Group protocol A5260s who initiated ritonavir-boosted darunavir (once daily with emtricitabine and tenofovir disoproxil fumarate) gained similar fat mass but statistically less lean mass 96 weeks following ART start compared to participants whose third ART agent was ritonavir-boosted atazanavir or raltegravir.[42] Additionally, cumulative darunavir use has been associated with incident cardiovascular disease,[43] and once daily ritonavir-boosted darunavir has been associated with more rapid increases in carotid intima-media thickness (a surrogate measure of cardiovascular disease burden) than ritonavir-boosted atazanavir or the unboosted integrase inhibitor raltegravir.[44] With similar changes in lipids, inflammatory parameters and fat distribution,[40, 42, 45] the findings of greater fat-to-lean mass gain, more rapid carotid intima media thickness progression and incident cardiovascular disease suggest that an unknown mechanism for the development of metabolic disease with darunavir may exist. Supporting this hypothesis is our finding that darunavir users had similar frequencies of traditional risk factors for metabolic disease as other PI users, and that the darunavir effect persisted after controlling for these factors.
This study has several limitations. First, our assessment of metabolic health status was cross-sectional. However, as noted in the methods, when comparing the most recent visit to metabolic health status over the last five years, results were comparable and using the most current visit allowed for presentation of data per person rather than in person-years. Second, the types of metabolic disturbances used to determine metabolic health status differed by HIV serostatus, with HIV-infected men more likely to meet criteria for lipid abnormalities. Acknowledging that the ATP III criteria may not be equivalent in their contributions to cardiometabolic outcomes, our next step is to conduct a longitudinal study to determine how the current observed differences in metabolic health affect clinical outcomes by HIV serostatus and BMI category.
Third, while this study was not designed to fully elucidate mechanisms of metabolic health or lack thereof, both traditional and HIV-specific factors significantly contributed to metabolic health status among HIV-infected men, with HIV-specific risk factors contributing disproportionately among normal weight and overweight, but not obese, HIV-infected men. Fourth, this analysis may not be generalizable to HIV-infected women or populations outside the US, although analyses in these populations are planned. Fifth, the ATP III criteria for MHO, while common in the literature and applicable to the majority of MACS men, do not incorporate waist circumference measurements that are race/ethnicity specific. Finally, we were unable to control for physician bias or regional differences in ART prescribing practices or comorbidity management, but our large sample size, detailed characterization and diverse geographic makeup are strengths of this analysis.
In conclusion, metabolically healthy obesity was similarly prevalent among HIV-infected and HIV-uninfected MACS men, with traditional risk factors most strongly influencing metabolic health status among obese men. However, HIV-infected normal weight and overweight men were much less likely than their HIV-uninfected counterparts to experience metabolic health, with both traditional risk factors and zidovudine and stavudine use associated with reduced likelihood of metabolic health among HIV-infected men. For HIV-infected men with greater than three years of zidovudine exposure, darunavir use was also associated with reduced likelihood of metabolic health. These findings emphasize the contributions of non-traditional risk factors for metabolic disease among non-obese, HIV-infected men, and demonstrate the importance of metabolic risk assessment across the BMI spectrum in this population.
Supplementary Material
Acknowledgments
The authors would also like to acknowledge the study participants for their generous participation.
Funding Sources
Data in this manuscript were collected by the MACS. MACS (Principal Investigators): Johns Hopkins University (JHU) Bloomberg School of Public Health (Joseph Margolick), U01-AI35042; Northwestern University (Steven Wolinsky), U01-AI35039; University of California, Los Angeles (Roger Detels), U01-AI35040; University of Pittsburgh (Charles Rinaldo), U01-AI35041; the Center for Analysis and Management of MACS, Johns Hopkins University Bloomberg School of Public Health (Lisa Jacobson), UM1-AI35043. The MACS is funded primarily by the National Institute of Allergy and Infectious Diseases, with additional co-funding from the National Cancer Institute, the National Institute on Drug Abuse, and the National Institute of Mental Health. Targeted supplemental funding for specific projects was also provided by the National Heart, Lung, and Blood Institute and the National Institute on Deafness and Communication Disorders. MACS data collection is also supported by UL1-TR001079 (JHU Institute for Clinical and Translational Research) from the National Center for Advancing Translational Sciences, a component of the National Institutes of Health (NIH), and the NIH Roadmap for Medical Research. The contents of this publication are solely the responsibility of the authors and do not represent the official views of the NIH, the JHU Institute for Clinical and Translational Research or the National Center for Advancing Translational Sciences. The MACS website is located at http://aidscohortstudy.org.
Additional research support provided by the National Institutes of Health grants U01 Al035040, R01 HL095129, UL1TR000124, P30 AG028748, K24 AI120834, K23 AG050260 and K23 AI110532-01A1 and the U.S. Department of Health and Human Services contract number HHSN272200800014C.
Footnotes
This data was presented in part at the 17th International Workshop on Co-morbidities and Adverse Drug Reactions in HIV in Barcelona, Spain, October 20–22, 2015.
DISCLOSURE STATEMENT
JEL has served as a consultant to Gilead Sciences and Merck, and has received research funding from Gilead.
KME has served as a consultant to Gilead Sciences and Theratechnologies, and has received research funding from Gilead.
LPJ has served as a consultant to Bristol Myers Squibb.
TTB has served as a consultant to AbbVie, EMD-Serono, Bristol Myers Squibb, Theratechnologies, Merck, ViiV Healthcare and Gilead.
XL, FJP Jr., DW and LK have no conflicts of interest to report.
Author Contributions
JEL designed the study, oversaw data analysis and interpretation, and was the primary author responsible for manuscript development.
KME, TTB, FJP Jr., DW and LK assisted with data analysis, data interpretation and manuscript revisions.
LPJ and XL oversaw and performed statistical analyses, respectively, and contributed to manuscript development.
References
- 1.Kim DJ, Westfall AO, Chamot E, Willig AL, Mugavero MJ, Ritchie C, et al. Multimorbidity patterns in HIV-infected patients: the role of obesity in chronic disease clustering. J Acquir Immune Defic Syndr. 2012;61(5):600–605. doi: 10.1097/QAI.0b013e31827303d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kroll AF, Sprinz E, Leal SC, Labrea Mda G, Setubal S. Prevalence of obesity and cardiovascular risk in patients with HIV/AIDS in Porto Alegre, Brazil. Arquivos brasileiros de endocrinologia e metabologia. 2012;56(2):137–141. doi: 10.1590/s0004-27302012000200007. [DOI] [PubMed] [Google Scholar]
- 3.Tate T, Willig AL, Willig JH, Raper JL, Moneyham L, Kempf MC, et al. HIV infection and obesity: where did all the wasting go? Antivir Ther. 2012;17(7):1281–1289. doi: 10.3851/IMP2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Isasti G, Perez I, Moreno T, Cabrera F, Palacios R, Santos J. Echocardiographic abnormalities and associated factors in a cohort of asymptomatic HIV-infected patients. AIDS research and human retroviruses. 2013;29(1):20–24. doi: 10.1089/AID.2012.0096. [DOI] [PubMed] [Google Scholar]
- 5.El-Sadr WM, Mullin CM, Carr A, Gibert C, Rappoport C, Visnegarwala F, et al. Effects of HIV disease on lipid, glucose and insulin levels: results from a large antiretroviral-naive cohort. HIV Med. 2005;6(2):114–121. doi: 10.1111/j.1468-1293.2005.00273.x. [DOI] [PubMed] [Google Scholar]
- 6.Tripathi A, Liese AD, Jerrell JM, Zhang J, Rizvi AA, Albrecht H, et al. Incidence of diabetes mellitus in a population-based cohort of HIV-infected and non-HIV-infected persons: the impact of clinical and therapeutic factors over time. Diabetic medicine : a journal of the British Diabetic Association. 2014 doi: 10.1111/dme.12455. [DOI] [PubMed] [Google Scholar]
- 7.Lake JE, Currier JS. Metabolic disease in HIV infection. The Lancet Infectious diseases. 2013;13(11):964–975. doi: 10.1016/S1473-3099(13)70271-8. [DOI] [PubMed] [Google Scholar]
- 8.Alam I, Ng TP, Larbi A. Does inflammation determine whether obesity is metabolically healthy or unhealthy? The aging perspective. Mediators of inflammation. 2012;2012:456456. doi: 10.1155/2012/456456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pathai S, Bajillan H, Landay AL, High KP. Is HIV a Model of Accelerated or Accentuated Aging? The journals of gerontology Series A, Biological sciences and medical sciences. 2014;69(7):833–842. doi: 10.1093/gerona/glt168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grundy SM, Brewer HB, Jr, Cleeman JI, Smith SC, Jr, Lenfant C, American Heart A et al. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109(3):433–438. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 11.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112(17):2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 12.Wildman RP, Muntner P, Reynolds K, McGinn AP, Rajpathak S, Wylie-Rosett J, et al. The obese without cardiometabolic risk factor clustering and the normal weight with cardiometabolic risk factor clustering: prevalence and correlates of 2 phenotypes among the US population (NHANES 1999–2004) Arch Intern Med. 2008;168(15):1617–1624. doi: 10.1001/archinte.168.15.1617. [DOI] [PubMed] [Google Scholar]
- 13.Sung KC, Cha SC, Sung JW, So MS, Byrne CD. Metabolically healthy obese subjects are at risk of fatty liver but not of pre-clinical atherosclerosis. Nutr Metab Cardiovasc Dis. 2014;24(3):256–262. doi: 10.1016/j.numecd.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 14.Phillips CM. Metabolically healthy obesity: definitions, determinants and clinical implications. Rev Endocr Metab Disord. 2013;14(3):219–227. doi: 10.1007/s11154-013-9252-x. [DOI] [PubMed] [Google Scholar]
- 15.Roberson LL, Aneni EC, Maziak W, Agatston A, Feldman T, Rouseff M, et al. Beyond BMI: The “Metabolically healthy obese” phenotype & its association with clinical/subclinical cardiovascular disease and all-cause mortality – a systematic review. BMC public health. 2014;14:14. doi: 10.1186/1471-2458-14-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Camhi SM, Katzmarzyk PT. Differences in body composition between metabolically healthy obese and metabolically abnormal obese adults. International journal of obesity. 2013 doi: 10.1038/ijo.2013.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hinnouho GM, Czernichow S, Dugravot A, Batty GD, Kivimaki M, Singh-Manoux A. Metabolically healthy obesity and risk of mortality: does the definition of metabolic health matter? Diabetes care. 2013;36(8):2294–2300. doi: 10.2337/dc12-1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khan UI, Wang D, Thurston RC, Sowers M, Sutton-Tyrrell K, Matthews KA, et al. Burden of subclinical cardiovascular disease in “metabolically benign” and “at-risk” overweight and obese women: the Study of Women’s Health Across the Nation (SWAN) Atherosclerosis. 2011;217(1):179–186. doi: 10.1016/j.atherosclerosis.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim TN, Park MS, Yang SJ, Yoo HJ, Kang HJ, Song W, et al. Body size phenotypes and low muscle mass: the Korean sarcopenic obesity study (KSOS) The Journal of clinical endocrinology and metabolism. 2013;98(2):811–817. doi: 10.1210/jc.2012-3292. [DOI] [PubMed] [Google Scholar]
- 20.Kramer CK, Zinman B, Retnakaran R. Are metabolically healthy overweight and obesity benign conditions?: A systematic review and meta-analysis. Annals of internal medicine. 2013;159(11):758–769. doi: 10.7326/0003-4819-159-11-201312030-00008. [DOI] [PubMed] [Google Scholar]
- 21.van der AD, Nooyens AC, van Duijnhoven FJ, Verschuren MM, Boer JM. All-cause mortality risk of metabolically healthy abdominal obese individuals: the EPIC-MORGEN study. Obesity (Silver Spring) 2014;22(2):557–564. doi: 10.1002/oby.20480. [DOI] [PubMed] [Google Scholar]
- 22.Shin MJ, Hyun YJ, Kim OY, Kim JY, Jang Y, Lee JH. Weight loss effect on inflammation and LDL oxidation in metabolically healthy but obese (MHO) individuals: low inflammation and LDL oxidation in MHO women. International journal of obesity. 2006;30(10):1529–1534. doi: 10.1038/sj.ijo.0803304. [DOI] [PubMed] [Google Scholar]
- 23.Lynch LA, O’Connell JM, Kwasnik AK, Cawood TJ, O’Farrelly C, O’Shea DB. Are natural killer cells protecting the metabolically healthy obese patient? Obesity. 2009;17(3):601–605. doi: 10.1038/oby.2008.565. [DOI] [PubMed] [Google Scholar]
- 24.Brochu M, Tchernof A, Dionne IJ, Sites CK, Eltabbakh GH, Sims EA, et al. What are the physical characteristics associated with a normal metabolic profile despite a high level of obesity in postmenopausal women? The Journal of clinical endocrinology and metabolism. 2001;86(3):1020–1025. doi: 10.1210/jcem.86.3.7365. [DOI] [PubMed] [Google Scholar]
- 25.Phillips CM, Perry IJ. Does inflammation determine metabolic health status in obese and nonobese adults? The Journal of clinical endocrinology and metabolism. 2013;98(10):E1610–1619. doi: 10.1210/jc.2013-2038. [DOI] [PubMed] [Google Scholar]
- 26.Primeau V, Coderre L, Karelis AD, Brochu M, Lavoie ME, Messier V, et al. Characterizing the profile of obese patients who are metabolically healthy. International journal of obesity. 2011;35(7):971–981. doi: 10.1038/ijo.2010.216. [DOI] [PubMed] [Google Scholar]
- 27.Paula AA, Falcao MC, Pacheco AG. Metabolic syndrome in HIV-infected individuals: underlying mechanisms and epidemiological aspects. AIDS Res Ther. 2013;10(1):32. doi: 10.1186/1742-6405-10-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaslow RA, Ostrow DG, Detels R, Phair JP, Polk BF, Rinaldo CR., Jr The Multicenter AIDS Cohort Study: rationale, organization, and selected characteristics of the participants. Am J Epidemiol. 1987;126(2):310–318. doi: 10.1093/aje/126.2.310. [DOI] [PubMed] [Google Scholar]
- 29.Brown TT, Chu H, Wang Z, Palella FJ, Kingsley L, Witt MD, et al. Longitudinal increases in waist circumference are associated with HIV-serostatus, independent of antiretroviral therapy. Aids. 2007;21(13):1731–1738. doi: 10.1097/QAD.0b013e328270356a. [DOI] [PubMed] [Google Scholar]
- 30.Hultin LE, Menendez FA, Hultin PM, Jamieson BD, O’Gorman MR, Borowski L, et al. Assessing immunophenotyping performance: proficiency-validation for adopting improved flow cytometry methods. Cytometry Part B, Clinical cytometry. 2007;72(4):249–255. doi: 10.1002/cyto.b.20176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brener MI, Post WS, Haberlen SA, Zhang L, Palella FJ, Jr, Jacobson LP, et al. Comparison of Insulin Resistance to Coronary Atherosclerosis in Human Immunodeficiency Virus Infected and Uninfected Men (from the Multicenter AIDS Cohort Study) Am J Cardiol. 2016;117(6):993–1000. doi: 10.1016/j.amjcard.2015.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Araujo S, Banon S, Machuca I, Moreno A, Perez-Elias MJ, Casado JL. Prevalence of insulin resistance and risk of diabetes mellitus in HIV-infected patients receiving current antiretroviral drugs. Eur J Endocrinol. 2014;171(5):545–554. doi: 10.1530/EJE-14-0337. [DOI] [PubMed] [Google Scholar]
- 33.Tomiyama AJ, Hunger JM, Nguyen-Cuu J, Wells C. Misclassification of cardiometabolic health when using body mass index categories in NHANES 2005–2012. Int J Obes (Lond) 2016;40(5):883–886. doi: 10.1038/ijo.2016.17. [DOI] [PubMed] [Google Scholar]
- 34.Goday A, Calvo E, Vazquez LA, Caveda E, Margallo T, Catalina-Romero C, et al. Prevalence and clinical characteristics of metabolically healthy obese individuals and other obese/non-obese metabolic phenotypes in a working population: results from the Icaria study. BMC Public Health. 2016;16:248. doi: 10.1186/s12889-016-2921-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Curran A, Ribera E. From old to new nucleoside reverse transcriptase inhibitors: changes in body fat composition, metabolic parameters and mitochondrial toxicity after the switch from thymidine analogs to tenofovir or abacavir. Expert Opin Drug Saf. 2011;10(3):389–406. doi: 10.1517/14740338.2011.542145. [DOI] [PubMed] [Google Scholar]
- 36.Kakuda TN. Pharmacology of nucleoside and nucleotide reverse transcriptase inhibitor-induced mitochondrial toxicity. Clinical therapeutics. 2000;22(6):685–708. doi: 10.1016/S0149-2918(00)90004-3. [DOI] [PubMed] [Google Scholar]
- 37.da Cunha J, Maselli LM, Stern AC, Spada C, Bydlowski SP. Impact of antiretroviral therapy on lipid metabolism of human immunodeficiency virus-infected patients: Old and new drugs. World J Virol. 2015;4(2):56–77. doi: 10.5501/wjv.v4.i2.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lv Z, Chu Y, Wang Y. HIV protease inhibitors: a review of molecular selectivity and toxicity. HIV AIDS (Auckl) 2015;7:95–104. doi: 10.2147/HIV.S79956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Overton ET, Arathoon E, Baraldi E, Tomaka F. Effect of darunavir on lipid profile in HIV-infected patients. HIV clinical trials. 2012;13(5):256–270. doi: 10.1310/hct1305-256. [DOI] [PubMed] [Google Scholar]
- 40.Ofotokun I, Na LH, Landovitz RJ, Ribaudo HJ, McComsey GA, Godfrey C, et al. Comparison of the metabolic effects of ritonavir-boosted darunavir or atazanavir versus raltegravir, and the impact of ritonavir plasma exposure: ACTG 5257. Clin Infect Dis. 2015;60(12):1842–1851. doi: 10.1093/cid/civ193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Deeks ED. Darunavir: a review of its use in the management of HIV-1 infection. Drugs. 2014;74(1):99–125. doi: 10.1007/s40265-013-0159-3. [DOI] [PubMed] [Google Scholar]
- 42.McComsey GA, Moser C, Currier J, Ribaudo HJ, Paczuski P, Dube MP, et al. Body Composition Changes After Initiation of Raltegravir or Protease Inhibitors: ACTG A5260s. Clin Infect Dis. 2016;62(7):853–862. doi: 10.1093/cid/ciw017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ryom L, L J, El-Sadr WM, Reiss P, Phillips A, Kirk O, Weber R, Sabin C, Mocroft A. Association between cardiovascular disease & contemporarily used protease inhibitors. 2017 [Google Scholar]
- 44.Stein JH, Ribaudo HJ, Hodis HN, Brown TT, Tran TT, Yan M, et al. A prospective, randomized clinical trial of antiretroviral therapies on carotid wall thickness. Aids. 2015;29(14):1775–1783. doi: 10.1097/QAD.0000000000000762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kelesidis T, Tran TT, Stein JH, Brown TT, Moser C, Ribaudo HJ, et al. Changes in Inflammation and Immune Activation With Atazanavir-, Raltegravir-, Darunavir-Based Initial Antiviral Therapy: ACTG 5260s. Clin Infect Dis. 2015;61(4):651–660. doi: 10.1093/cid/civ327. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


