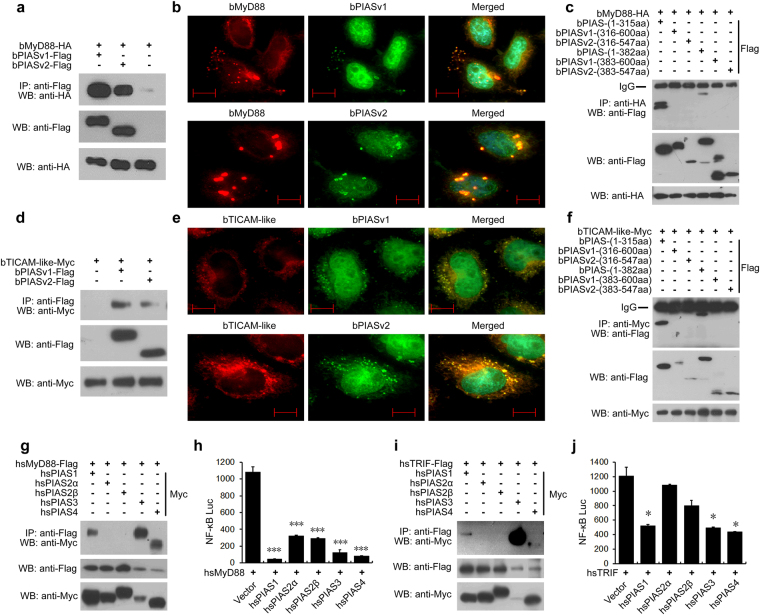
Figure 5.
Both amphioxus and human PIAS can bind to MyD88 and TICAM-like and suppress NF-κB activation. (a) Co-IP analyses of the interactions between bPIAS and bMyD88. (b) Immunofluorescence analysis of the subcellular co-localization of amphioxus PIAS and MyD88 in HeLa cells, which were co-transfected with EGFP-fused bPIASv1 or v2 with HA-tagged bMyD88 and stained with anti-HA antibody and an Alexa Fluor 532 secondary antibody. Scale bar indicates 10 μm. (c) Co-IP analyses of the interactions between the truncated mutants of bPIAS and bMyD88. (d) Co-IP analyses of the interactions between bPIAS and bTICAM-like. (e) Immunofluorescence analysis of the subcellular co-localization of amphioxus PIAS and bTICAM-like in HeLa cells, which were co-transfected with EGFP-fused bPIASv1 or v2 with Myc-tagged bTICAM-like and stained with anti-Myc antibody and an Alexa Fluor 532 secondary antibody. The scale bar indicates 10 µm. (f) Co-IP analyses of the interactions between the truncated mutants of bPIAS and bTICAM-like. (g) Co-IP analyses of the interactions between human PIAS and MyD88. (h) Luciferase reporter assays indicate that human PIAS1, 3 and 4 can attenuate hsMyD88-induced NF-κB activation more robustly. (i) Co-IP analyses of the interactions between human PIAS and TRIF. (j) Luciferase reporter assays show that human PIAS1, 3 and 4 but not PIAS2 can repress NF-κB activation mediated by hsTRIF. All experiments were conducted at least twice. Data in (h) and (j) are means ± SD for 3 independent experiments. *P < 0.05, ***P < 0.001.