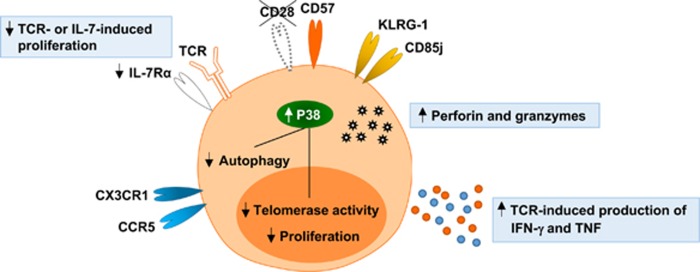
Memory T cells undergo replicative senescence via repetitive proliferation. As in other cells, replicative senescence of T cells is characterized by shortened telomeres.1 Immunophenotypes are also altered in replicative senescent T cells compared to young T cells. In humans, replicative senescent T cells typically lose expression of CD28, a co-stimulatory receptor, and acquire the expression of CD57 antigen, a terminally sulfated glycan carbohydrateepitope,1 though the mechanism underlying this regulation has not yet been clearly elucidated. Therefore, CD28− or CD57+ T cells are considered replicative senescent T cells in humans, and the frequency of CD28− or CD57+ T cells in the peripheral blood increases with aging.2, 3
Replicative senescent CD28− or CD57+ T cells gain the expression of NK cell-related receptors, such as KLRG-1 and CD85j, and chemokine receptors for homing to peripheral inflammatory sites, such as CX3CR1 and CCR5 (Figure 1).1, 4 Moreover, CD28− or CD57+ T cells express higher levels of cytotoxic proteins, such as perforin and granzyme B, and produce higher amounts of IFN-γ and tumor necrosis factor upon T cell receptor (TCR) stimulation compared to non-senescent CD28+ or CD57− T cells (Figure 1).5 In terms of cell proliferation, CD28− or CD57+ T cells are less responsive to TCR or IL-7 stimulation than their CD28+ or CD57− counterparts (Figure 1).1, 4 In particular, CD28− or CD57+ T cells express reduced levels of IL-7 receptor α-chain (CD127). However, IL-15-induced proliferation is intact in CD28− or CD57+ T cells. Furthermore, senescent T cells are different from non-senescent T cells in regards to intracellular signaling. In senescent human T cells, the mitogen-activated protein kinase (MAPK) p38 pathway is activated by intracellular changes, leading to inhibition of autophagy, proliferation and telomerase activity (Figure 1).6, 7, 8 An immune-inhibitory role of the sestrin–MAPK activation complex in senescent T cells was also reported recently.9
Figure 1.
Characteristics of replicative senescent T cells. Replicative senescent T cells lose the expression of CD28 and acquire the expression of CD57, NK cell-related receptors, and chemokine receptors for peripheral homing. They have high levels of perforin and granzymes, and produce high amounts of IFN-γ and TNF upon TCR stimulation. However, these cells are less proliferative to TCR or IL-7 stimulation compared to young T cells. They exhibit constitutive p38 mitogen-activated protein kinase (MAPK) activation, leading to inhibition of autophagy, proliferation, and telomerase activity. TCR; T cell receptor, TNF; tumor necrosis factor.
In principle, replicative senescence of T cells can be induced by any antigen or microbial pathogen if it is encountered repetitively. Among various human pathogens, cytomegalovirus (CMV) is the major virus responsible for the accumulation of replicative senescent CD28− or CD57+ T cells in humans.10, 11 CMV is a human herpesvirus that latently infects a majority of the population. CMV usually infects individuals asymptomatically during early childhood and establishes a lifelong latent infection,10, 11 but it is occasionally reactivated even in non-immunocompromised healthy hosts depending on the immune status of the host over their lifetime. Repetitive cycles of CMV reactivation and resolution result in repetitive antigenic stimulation of CMV-specific memory T cells and consequent accumulation of replicative senescent CMV-specific T cells over a person’s lifetime.10, 11, 12 A sustained increase and maintenance of CMV-specific memory T cells over a lifetime is known as ‘memory inflation’.10, 12
Possible roles of replicative senescent T cells in human cardiovascular disease were first reported in patients with unstable angina.13 The number of CD4+CD28− T cells in the peripheral blood was significantly increased in patients with unstable angina compared to patients with chronic stable angina,13 and a higher frequency of CD4+CD28− T cells was significantly associated with the recurrence of acute coronary events in patients with unstable angina.14 In addition, accelerated telomere shortening was demonstrated in T cells from patients with atherosclerosis.15 Senescent T cells were also reported to be important in human hypertension. The relative frequency of CD57+ or CD28− cells in the peripheral CD8+ T-cell population was increased in patients with essential hypertension compared to age- and sex-matched control subjects.16 Interestingly, increased expression of CXCL11 and T-cell infiltration were observed in renal tissues from patients with hypertension. Senescent CD8+ T cells are also associated with the prognosis of acute myocardial infarction (MI). The relative frequency of CD57+ cells in the peripheral CD8+ T-cell population has been shown to significantly correlate with cardiovascular mortality 6 months after acute MI.17
Recently, an association between replicative senescence of T cells and arterial stiffness has been demonstrated in healthy individuals.18 Arterial stiffness is increased in the presence of cardiovascular risk factors, including aging, and known to be associated with inflammation. In this study, the relative frequency of CD57+cells in the peripheral CD8+ T-cell population independently correlated with arterial stiffness after adjusting for traditional cardiovascular risk factors.18 More importantly, the relative frequency of CMV pp65-specific CD8+ T cells in the peripheral CD8+ T-cell population, which was evaluated by the production of IFN-γ or tumor necrosis factor and CD107a exposure upon CMV pp65 stimulation, independently correlated with arterial stiffness.18 This study first demonstrated that arterial stiffness, representing vascular aging, is associated with senescent CD8+CD57+ T cells and CMV pp65-specific CD8+T cells.
As described above, there is a growing body of evidence indicating that replicative senescent CD28− or CD57+ T cells are involved in the development and progression of cardiovascular diseases, such as atherosclerosis, acute MI and hypertension, in humans. However, how replicative senescent T cells are regulated and contribute to the pathogenesis of cardiovascular diseases remains to be elucidated. For example, it is not yet clear whether senescent CD8+ and CD4+ T cells exert distinct functions in cardiovascular disease. In addition, it is important to determine how T-cell effector functions are altered during replicative senescence, which effector function of senescent T cells has a major role in the pathogenesis of cardiovascular diseases, how their effector functions are stimulated in the presence or absence of cognate antigens, and which tissue is the primary site for senescent T-cell infiltration and action. By answering these questions, we will be able to develop better strategies for the prevention and treatment of cardiovascular diseases.
Footnotes
The authors declare no conflict of interest.
References
- Akbar AN, Henson SM, Lanna A. Senescence of T lymphocytes: implications for enhancing human immunity. Trends Immunol 2016; 37: 866–876. [DOI] [PubMed] [Google Scholar]
- Focosi D, Bestagno M, Burrone O, Petrini M. CD57+ T lymphocytes and functional immune deficiency. J Leukoc Biol 2010; 87: 107–116. [DOI] [PubMed] [Google Scholar]
- Weng NP, Akbar AN, Goronzy J. CD28(−) T cells: their role in the age-associated decline of immune function. Trends Immunol 2009; 30: 306–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira BI, Akbar AN. Convergence of innate and adaptive immunity during human aging. Front Immunol 2016; 7: 445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu HT, Park S, Shin EC, Lee WW. T cell senescence and cardiovascular diseases. Clin Exp Med 2016; 16: 257–263. [DOI] [PubMed] [Google Scholar]
- Henson SM, Lanna A, Riddell NE, Franzese O, Macaulay R, Griffiths SJ et al. p38 signaling inhibits mTORC1-independent autophagy in senescent human CD8(+) T cells. J Clin Invest 2014; 124: 4004–4016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson SM, Macaulay R, Riddell NE, Nunn CJ, Akbar AN. Blockade of PD-1 or p38 MAP kinase signaling enhances senescent human CD8(+) T-cell proliferation by distinct pathways. Eur J Immunol 2015; 45: 1441–1451. [DOI] [PubMed] [Google Scholar]
- Lanna A, Henson SM, Escors D, Akbar AN. The kinase p38 activated by the metabolic regulator AMPK and scaffold TAB1 drives the senescence of human T cells. Nat Immunol 2014; 15: 965–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanna A, Gomes DC, Muller-Durovic B, McDonnell T, Escors D, Gilroy DW et al. A sestrin-dependent Erk-Jnk-p38 MAPK activation complex inhibits immunity during aging. Nat Immunol 2017; 18: 354–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Kim AR, Shin EC. Cytomegalovirus infection and memory T cell inflation. Immune Netw 2015; 15: 186–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolich-Zugich J. Ageing and life-long maintenance of T-cell subsets in the face of latent persistent infections. Nat Rev Immunol 2008; 8: 512–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klenerman P, Oxenius A. T cell responses to cytomegalovirus. Nat Rev Immunol 2016; 16: 367–377. [DOI] [PubMed] [Google Scholar]
- Liuzzo G, Kopecky SL, Frye RL, O'Fallon WM, Maseri A, Goronzy JJ et al. Perturbation of the T-cell repertoire in patients with unstable angina. Circulation 1999; 100: 2135–2139. [DOI] [PubMed] [Google Scholar]
- Liuzzo G, Biasucci LM, Trotta G, Brugaletta S, Pinnelli M, Digianuario G et al. Unusual CD4+CD28null T lymphocytes and recurrence of acute coronary events. J Am Coll Cardiol 2007; 50: 1450–1458. [DOI] [PubMed] [Google Scholar]
- Samani NJ, Boultby R, Butler R, Thompson JR, Goodall AH. Telomere shortening in atherosclerosis. Lancet 2001; 358: 472–473. [DOI] [PubMed] [Google Scholar]
- Youn JC, Yu HT, Lim BJ, Koh MJ, Lee J, Chang DY et al. Immunosenescent CD8+ T cells and C-X-C chemokine receptor type 3 chemokines are increased in human hypertension. Hypertension 2013; 62: 126–133. [DOI] [PubMed] [Google Scholar]
- Tae YuH, Youn JC, Lee J, Park S, Chi HS, Lee J et al. Characterization of CD8(+)CD57(+) T cells in patients with acute myocardial infarction. Cell Mol Immunol 2015; 12: 466–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu HT, Youn JC, Kim JH, Seong YJ, Park SH, Kim HC et al. Arterial stiffness is associated with cytomegalovirus-specific senescent CD8+ T cells. J Am Heart Assoc 2017; 6: e006535. [DOI] [PMC free article] [PubMed] [Google Scholar]