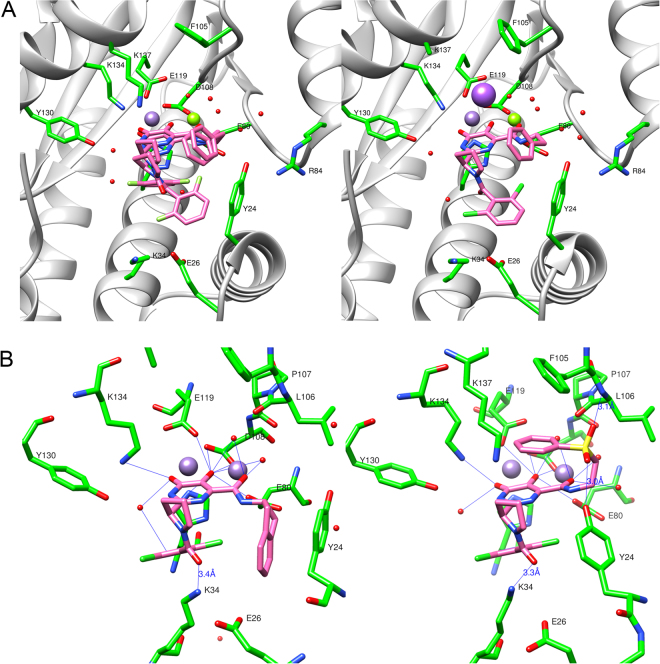
Figure 3.
The wild type PAN high affinity bound conformations of inhibitors from high-resolution co-crystal structures. (A) The PAN inhibitor co-crystal structures of 7b (left panel, which shows the cis (44%) and trans (56%) bound conformations) and 7a (right panel, which shows the trans bound conformation). (B) The bound conformation of 8f (left panel) in the PAN active site and the bound conformation of 8e (right panel) in the wild type PAN construct showing potential hydrogen-bond interactions with crystallographic water molecules, active-site side chains, and a main chain interaction. Both structures feature H-bond donation from Lys34 to the pyrrolidine amide carbonyl (3.4 Å and 3.3 Å, respectively), and numerous interactions near the two-metal site, which are common to all DHPC inhibitors. In 8e, note the main chain (Leu106) H-bond (3.1 Å) to one sulfone oxygen and the H-bond (3.0 Å) donation from Tyr24 the other sulfone oxygen. Mn2++ ions are shown as violet spheres and Mg2++ as green spheres. See Supporting Figure S1b for electron density maps for examples of these and other relevant structures.