Fig. 6.
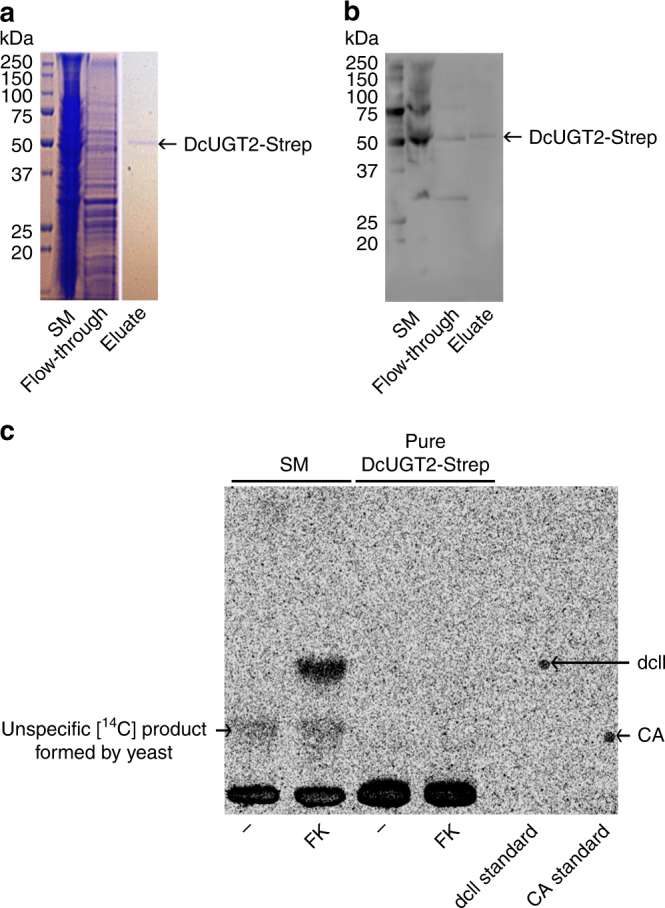
DcUGT2-Strep loses enzyme activity upon affinity purification. Yeast microsomes containing DcUGT2-Strep were solubilized with reduced Triton X-100 and DcUGT2 affinity purified by its Strep-tag II. Protein fractions were tested for glucosylation activity by incubation with [14C]UDP-glucose and with/without flavokermesic acid. a Protein samples separated on an SDS gel followed by Coomassie staining. b Protein samples separated on an SDS gel followed by western blotting using an anti-Strep antibody. c TLC-separated [14C] products formed in vitro and viewed by phosphorimaging. SM solubilized microsomes; flow-through proteins that did not bind to the affinity matrix; eluate protein fraction C1 (Supplementary Fig. 3) which was eluted from the affinity matrix with desthiobiotin. FK flavokermesic acid, CA carminic acid. Note that the anti-Strep antibody reacts with the marker protein bands
