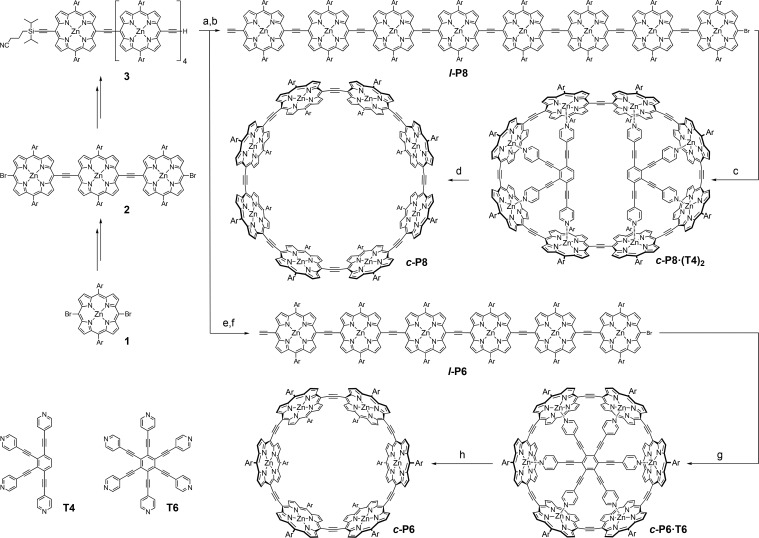
Scheme 1. Synthesis of c-P8 and c-P6.
Reaction conditions: (a) 2, Pd2dba3, PPh3, CuI, PhMe/i-Pr2NH (2:1), 50 °C, 15 h, 13%; (b) TBAF, CH2Cl2, 25 °C, 10 min, 79%; (c) T4, Pd2dba3, PPh3, CuI, PhMe/i-Pr2NH (10:1), 40 °C, 2 d, 32%; (d) SEC pyridine/PhMe (1:1), 99%; (e) 1, Pd(PPh3)4, PPh3, CuI, PhMe/i-Pr2NH (2:1), 50 °C, 15 h, 30%; (f) TBAF, CH2Cl2, 25 °C, 10 min, 95%; (g) T6, Pd(PPh3)4, PPh3, CuI, PhMe/i-Pr2NH (2:1), 40 °C, 16 h, 15%; (h) SEC (pyridine/PhMe 1:1), 99%. Ar: 1,3-bis(trihexylsilyl)phenyl. Syntheses of 1, 2, 3, T4 and T6 are outlined in the SI.