FIGURE 1.
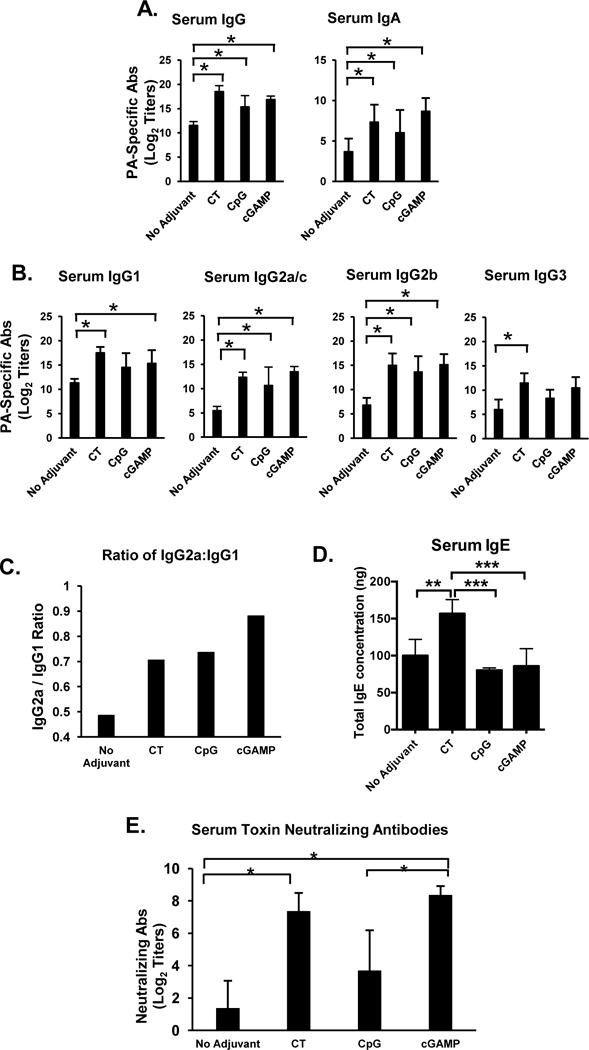
Sublingual immunization with the STING ligand 3′3′-cGAMP as adjuvant enhances systemic antibody responses. Mice were immunized by sublingual application of PA alone, PA plus cholera toxin (CT), PA plus CpG ODN 1826 (CpG), or PA plus the STING ligand 3′3′-cGAMP (3′3′-cGAMP), three times at weekly intervals. Serum samples were collected 1 week (Day 21) after the last immunization and PA-specific Ab responses were analyzed by ELISA. (A) PA-specific serum IgG and IgA Ab responses; (B) PA-specific serum IgG subclass (IgG1, IgG2a, IgG2b and IgG3) responses; (C) Ratio of IgG2a/IgG1 Ab responses. Data are expressed as mean Ab titers ± SD (n=6 per group). (D) IgE Ab responses. Data are expressed as mean concentration of total IgE ± SD (n=6 per group). (E) PA-specific neutralizing Ab titers were determined by the in vitro toxicity assay. Neutralizing Ab titers are expressed as mean ± SD (n=3 per group). *p ≤ 0.05 compared to the PA only (no adjuvant) group (Dunnett’s post-test).
