Abstract
Self-management (SM) is defined as the provision of interventions to increase patients’ skills and confidence, empowering the individual to take an active part in their disease management. There is uncertainty regarding the optimal format and the short- and long-term benefits of chronic obstructive pulmonary disease (COPD) SM interventions in adults. Therefore, a high-quality overview of reviews was updated to examine their clinical effectiveness. Sixteen reviews were identified, interventions were broadly classified as education or action plans, complex interventions with an SM focus, pulmonary rehabilitation (PR), telehealth and outreach nursing. Systematic review and meta-analysis quality and the risk of bias of underlying primary studies were assessed. Strong evidence was found that PR is associated with significant improvements in health-related quality of life (HRQoL). Limited to moderate evidence for complex interventions (SM focus) with limited evidence for education, action plans, telehealth interventions and outreach nursing for HRQoL was found. There was strong evidence that education is associated with a significant reduction in COPD-related hospital admissions, moderate to strong evidence that telehealth interventions and moderate evidence that complex interventions (SM focus) are associated with reduced health care utilization. These findings from a large body of evidence suggesting that SM, through education or as a component of PR, confers significant health gains in people with COPD in terms of HRQoL. SM supported by telehealth confers significant reductions in healthcare utilization, including hospitalization and emergency department visits.
Keywords: Self-management, self-management interventions, COPD, chronic disease, overview of review, systematic review
Introduction
The World Health Organization predicts that by 2030 chronic obstructive pulmonary disease (COPD) will be the third leading cause of death globally.1 There is no cure currently, but proactive management can improve health outcomes. In the later stages of the disease, health service use often increases with frequent hospitalizations.
SM is defined in general as ‘the systematic provision of education and supportive interventions by health care staff to increase patients’ skills and confidence in managing their health problems, including regular assessment of progress and problems, goal setting, and problem-solving support’.2
SM interventions to enhance SM skills and to improve self-efficacy are very broad, which may be due to the lack of an agreed definition until very recently. In 2012, the UK National Institute for Health Research commissioned the PRISMS study, an overview of reviews of SM support interventions, published in 2014. PRISMS proposed SM support taxonomy (not specifically for COPD), which includes four ‘overarching dimensions’ to aid description and characterization – delivery mode; personnel delivering the support; intervention targeting; and the intensity, frequency and duration of the intervention.3 A recent agreed definition of COPD SM interventions by an international expert group states that ‘a COPD SM intervention is structured but personalized and often multi-component, with goals of motivating, engaging and supporting the patients to positively adapt their health behaviour(s) and develop skills to better manage their disease’.4 They state that the ultimate goals of SM include optimizing and preserving physical health; reducing symptoms and functional impairments in daily life and increasing emotional well-being, social well-being and quality of life; and establishing effective alliances with healthcare professionals, family, friends and community.4 They also state that iterative interactions with healthcare professionals focus on identifying needs, eliciting goals, formulating strategies and re-evaluating these as necessary. An agreed definition of COPD SM interventions by an international expert group is an important step forward; however, the benefits of this agreed definition and proposed taxonomy may not be known for some time until they are fully and uniformly adopted.
To date, a large volume of literature has been published on COPD SM interventions.5 However, uncertainty regarding optimal format and short- and long-term benefits still exists presenting an obstacle to informed decision-making about COPD SM provision. In addition, SM interventions are evolving rapidly, and it is therefore timely to update the PRISMS overview. This study aimed to determine the clinical effectiveness of SM interventions for adults with COPD. The outcomes of interest are health-related quality of life (HRQoL), health care utilization and mortality.
Methods
A scoping review identified a large body of clinical effectiveness literature including multiple systematic reviews evaluating a range of SM interventions in chronic diseases. We adopted an ‘overview of reviews’ approach, a term used by the Cochrane Library, described as an efficient method to systematically gather evidence and provide broad statements on the effectiveness of interventions.6 It is particularly useful for informing health service policy and delivery.7,8 A high-quality overview of reviews, PRISMS study, was used as a basis3 and the results were combined to give an updated overview of the available evidence.
Search strategy and study selection
The systematic overview was conducted in accordance with the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines.9 The search was carried out using PubMed, Embase and the Cochrane Library with results retrieved from January 2013 to 1 April 2015. January 2013 was selected to coincide with the practical systematic reviews in self-management support (PRISMS) search end date (January 1993 to January 2013). Our search was undertaken as part of a wider study of chronic disease SM interventions where a broader search string was used. Chronic disease terms including ‘COPD’ terms were combined with ‘self-management’ and ‘systematic review’ terms or filters in a sensitive search strategy (Table 1). Citation lists of included studies and relevant systematic reviews were scanned. Cochrane reviews included in PRISMS were checked for updates. Reviews in English were included.
Table 1.
Systematic search strategy and terms.
| Database | Search terms |
|---|---|
| PubMed and Embase |
|
| The Cochrane Library |
|
SM: self-management; SMS: short message service; COPD: chronic obstructive pulmonary disease.
Participants, Interventions, Comparators, Outcomes and Study design (PICOS)
Systematic reviews of randomized controlled trials (RCTs) comparing SM interventions with routine care for adults (≥18 years) with COPD were included. Any systematic reviews of SM interventions that helped patients to manage aspects of their COPD through education, training and support were included. With the exception of education interventions or action plans, this review did not assess single component SM (e.g. text message appointment reminders). All formats and delivery methods were considered (including telehealth, not a specific focus of PRISMS and are therefore included from 2009 onwards. This is discussed further in the Discussion section). Pulmonary rehabilitation (PR) was considered to include SM if it encompassed, for example, providing information about COPD; building skills like goal setting, problem solving and decision-making.10 The primary outcomes of interest were HRQoL, health care utilization and mortality.
Data extraction and quality assurance
Preliminary screening of all returned results was performed by a single person to eliminate clearly irrelevant studies. Two reviewers independently screened titles, abstracts and then full texts using the defined inclusion and exclusion criteria. Disagreements were resolved by discussion. Data extraction was performed independently by two people, with disagreements resolved by discussion.
Quality assessment of the systematic reviews was carried out by two people independently using the Revised Assessment of Multiple Systematic Reviews (R-AMSTAR) quality appraisal tool (minimum score = 11, maximum = 44).11 Reviews were categorized as higher quality (≥31), lower quality (<31) and lower impact (participants <1000). Participant numbers do not affect the quality of the systematic review process but provide information about the volume of evidence. We used these to weigh our interpretation of the evidence per review (Table 2).5,7
Table 2.
Quality assurance of systematic reviews and evidence of effect.a
| Quality of systematic reviews | ||||
|---|---|---|---|---|
| Quality of systematic review (R-AMSTAR) | Systematic review sample size | Overall value | ||
| Lower quality (R-AMSTAR score <31) | Smaller sample size (<1000 participants) | Low/Low | ||
| Lower quality (R-AMSTAR score <31) | Larger sample size (≥1000 participants) | Low/High | ||
| Higher quality (R-AMSTAR score ≥31) | Smaller sample size (<1000 participants) | High/Low | ||
| Higher quality (R-AMSTAR score ≥31) | Larger sample size (≥1000 participants) | High/High | ||
| Evidence of effect | ||||
| 0 | p > 0.05 | No evidence of effect | ||
| +/− | 0.05 ≥ p > 0.01 | Some evidence of effect in favour of intervention/control | ||
| ++/− − | 0.01 ≥ p > 0.001 | Strong evidence of effect in favour of intervention/control | ||
| +++/− − − | p ≤ 0.001 | Very strong evidence of effect in favour of intervention/control | ||
R-AMSTAR: Revised Assessment of Multiple Systematic Reviews.
aTable adapted from PRISMS study.
Data from the primary RCTs included in the systematic reviews was extracted, where available,6 from all individual RCTs as this information was not included in the PRISMS study. The reviews were categorized as being at high (>50% of included RCTs at high risk of bias) or low risk of bias (≤50% at high risk of bias). If a meta-analysis was undertaken, the methodological quality was categorized into high, medium and low quality using Higgins et al.’s quality assessment tool to facilitate interpretation of findings (Table 2).7
SM interventions were classified into broad intervention types based mainly on the reviews retrieved, the PRISMS study and taxonomy to facilitate comparison. RCT crossover between reviews was assessed as it could affect interpretation of results. If there was substantial crossover between two reviews (>70%), if one study was deemed better quality (R-AMSTAR score) and was published the same year or more recently, then the results of the better quality review were included. Otherwise, results from both reviews were included.
A narrative approach was used to synthesize the available evidence (meta-analysis is not appropriate at overview level due to RCT crossover). To aid interpretation, we broadly categorized the evidence into ‘strong’, ‘moderate’ and ‘limited’ strength of evidence based on the quality of the evidence and the statistical significance of the results (Table 2).
Results
Study detail
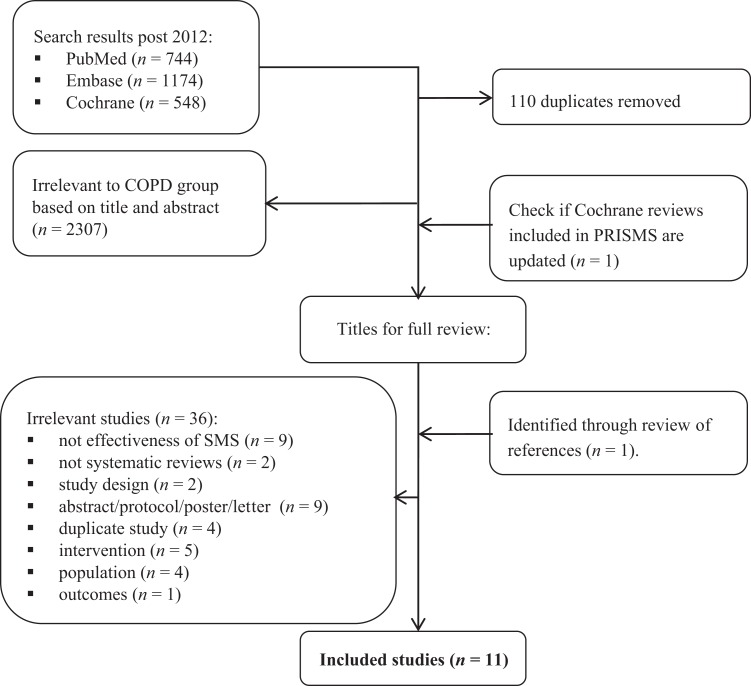
Sixteen systematic reviews were retrieved that assessed a diverse range of COPD SM interventions including education and action plans, complex interventions with a focus on SM, PR and interventions delivered using telehealth (Figure 1; Table 3). Five reviews were identified in PRISMS and a further 11 as part of this study. Publication dates ranged from 2005 to 2015 (systematic reviews) and from 1977 to 2013 (included RCTs). The number of included RCTs per systematic review ranged from 314 to 6522 and the number of participants from 36714 to 3941.17 RCT study locations were typically in Europe or North America. The 16 reviews included a total of 165 unique RCTs with approximately 16,500 participants; see Table 4 for study crossover. Results from the reviews by Harrison et al.18 and Bentsen et al.16 are not discussed further due to large study crossover (>70%) with another higher quality review by Jordan et al.21 and Zwerink et al.,20 respectively (Tables 5 and 6).
Figure 1.
PRISMA flow diagram of updated search results. Note: PRISMS retrieved a further five studies.
Table 3.
Quality appraisal of included studies.
| Study (Year) | Intervention | Systematic reviews | Primary studies | |||||
|---|---|---|---|---|---|---|---|---|
| R-AMSTAR score | Participants | Summarya | RCTs (n) | Low-risk of biasc | Meta-analysis quality | Summaryb | ||
| Education/action plans | ||||||||
| Effing et al. 12 e | Self-management education with or without action plans | 34 | 2239 | High/High | 13 | 3 | High | High/High |
| Tan et al. 13 | Self-management education | 33 | 2103 | High/High | 12 | 2 | High | High/High |
| Turnock et al.14 d | Action plans | 39 | 367 | High/Low | 3 | 0 | High | High/High |
| Walters et al.15 d | Action plans – COPD exacerbations | 34 | 574 | High/Low | 5 | 1 | High | High/High |
| Complex interventions with an SM focus | ||||||||
| Bentsen et al.16 | Range of SM interventions | 26 | 529 | Low/Low | 4 | NR | NA | NA |
| Dickens et al.17 | Range of complex interventions | 35 | 3941 | High/High | 32 | 8 | Medium | High/Med. |
| Harrison et al.18 | Range of SM – following COPD exacerbation | 30 | 1115 | Low/High | 7 | NR | NA | NA |
| Kruis et al.19 | Range of IDM interventions | 37 | 2997 | High/High | 26 | 5 | High | High/High |
| Zwerink et al.20e | Range of SM interventions | 39 | 3688 | High/High | 29 | 9 | High | High/High |
| Jordan et al.21 | Range of SM – following exacerbation | 40 | 1502 | High/High | 10 | 1 | High | High/High |
| Pulmonary rehabilitation | ||||||||
| McCarthy et al.22 | Pulmonary rehabilitation | 41 | 3822 | High/High | 65 | 17 | High | High/High |
| Telehealth | ||||||||
| Cruz et al.23 | Home telemonitoring | 33 | 587 | High/Low | 9 | 2 | High | High/High |
| Kamei et al.24 | Telehome monitoring-based telenursing | 30 | 550 | Low/Low | 9 | 6 | Medium | Low/Med. |
| Lundell et al.25 | Telehealth – making pulmonary rehabilitation accessible | 36 | 982 | High/Low | 9 | 2 | Low | High/Low |
| McLean et al.26 | Telehealth | 39 | 1004 | High/High | 10 | 0 | High | High/High |
| Outreach nursing programmes | ||||||||
| Wong et al.27 | Home care by outreach nursing | 37 | 1498 | High/High | 9 | 4 | High | High/High |
R-AMSTAR: Revised Assessment of Multiple Systematic Reviews; COPD: chronic obstructive pulmonary disease; IDM: integrated disease management; RCTs: randomized controlled trials; SM: self-management; NR: not reported; NA: not applicable.
aSummary includes R-AMSTAR score/number of participants. Papers judged to be of higher quality if scored ≥31 and lower quality if scored <31. Reviews judged to be of lower impact if total participant numbers are fewer than 1000.
bSummary includes risk of bias/meta-analysis quality score. If >50% of the included RCTs were at high risk of bias, review is rated as high risk of bias. The meta-analysis quality was evaluated using Higgins et al.’s quality assessment tool and results categorized into high-, medium- and low-quality meta-analysis.
cNumber of the total primary studies identified as being at low risk of bias.
dWalter’s Cochrane review (CR) is an update of Turnock’s CR.
eZwerink’s CR is an update of Effing’s CR. Note: In Zwerink’s update, they chose to exclude studies with education as the only active intervention.
Table 4.
Study crossover between the included systematic reviews.a
| Review (year) | A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | Bentsen et al.16 | 4 | |||||||||||||||
| B | Effing et al.12 | 2 | 13 | ||||||||||||||
| C | Tan et al.12 | 2 | 4 | 12 | |||||||||||||
| D | Turnock et al.14 | 0 | 1 | 1 | 3 | ||||||||||||
| E | Wong et al.27 | 1 | 4 | 2 | 0 | 9 | |||||||||||
| F | Cruz et al.23 | 0 | 0 | 0 | 0 | 0 | 10 | ||||||||||
| G | Dickens et al.17 | 1 | 5 | 4 | 1 | 4 | 1 | 32 | |||||||||
| H | Harrison et al.18 | 0 | 0 | 2 | 0 | 1 | 0 | 4 | 7 | ||||||||
| I | Kamei et al.24 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 7 | |||||||
| J | Kruis et al.19 | 1 | 4 | 2 | 0 | 4 | 1 | 1 | 1 | 0 | 26 | ||||||
| K | Lundell et al.25 | 1 | 1 | 2 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 9 | |||||
| L | Zwerink et al.20 | 3 | 6 | 5 | 0 | 2 | 1 | 7 | 1 | 0 | 6 | 2 | 29 | ||||
| M | McLean et al.26 | 1 | 1 | 2 | 0 | 1 | 0 | 3 | 3 | 2 | 1 | 3 | 3 | 10 | |||
| N | Walters et al.15 | 0 | 2 | 1 | 3 | 0 | 0 | 3 | 1 | 0 | 1 | 0 | 0 | 0 | 5 | ||
| O | Jordan et al.21 | 0 | 0 | 2 | 0 | 2 | 0 | 4 | 5 | 0 | 1 | 1 | 1 | 2 | 0 | 10 | |
| P | McCarthy et al.22 | 0 | 3 | 0 | 0 | 1 | 0 | 3 | 0 | 0 | 12 | 0 | 2 | 0 | 0 | 1 | 65 |
aPRISMS study is based on a search from 1993 to January 2013. This search was updated to April 2015.
Table 5.
Summary of findings from meta-analysis of impact of SM interventions.
| Study | HRQoL | Hospitalization | ED visits | Mortality |
|---|---|---|---|---|
| Education/action plans | ||||
| Effing et al.12 | WMD −2.58 (−5.14 to 0.02) | OR 0.64 (0.47 to 0.89) | NR | NR |
| Tan et al.13 | WMD −3.78 (−6.82 to −0.73) | OR 0.55 (0.43 to 0.71) | NR | NR |
| Turnock et al.14 | WMD −0.32 (−3.34 to 2.70) | b | NR | NR |
| Walters et al.15 | WMD −0.54 (−3.05 to 1.98) | b | NR | NR |
| Complex interventions with an SM focus | ||||
| Dickens et al.17 | NR | NR | OR 0.68 (0.57 to 0.80) | NR |
| Kruis et al.19 | WMD −0.22 (−7.43 to 6.99)c | OR 0.59 (0.28 to 1.22) | OR 0.64 (0.33 to 1.25) | OR 0.45 (0.16 to 1.28) |
| Zwerink et al.20 | WMD −3.51 (−5.37 to −1.65) | OR 0.57 (0.43 to 0.75) | NR | OR 0.79 (0.58 to 1.07) |
| Jordan et al.21 | WMD −3.84 (−6.40 to −1.29) | HR 0.78 (0.52 to 1.17) | Not combined, low quality | HR 1.07 (0.74 to 1.54) |
| Pulmonary rehabilitation | ||||
| McCarthy et al.22 | WMD −6.89 (−9.26 to −4.52)d | NR | NR | NR |
| Telehealth | ||||
| Cruz et al.23 | SMD −0.53 (−0.97 to −0.09) | RR 0.72 (0.53 to 0.98) | RR 0.68 (0.38 to 1.18) | RR 1.43 (0.40 to 5.03) |
| Kamei et al.24 | NR | RR 0.80 (0.68 to 0.94) | RR 0.52 (0.41 to 0.65) | RR 1.36 (0.77 to 2.41) |
| Lundell et al.25 | NR | NR | NR | NR |
| McLean et al.26 | WMD −6.57(−13.62 to −0.48) | OR 0.46 (0.33 to 0.65) | OR 0.27 (0.11 to 0.66) | NR |
| Outreach nursing programme | ||||
| Wong et al.27 | WMD −2.60 (−4.81 to −0.39) | OR 1.01 (0.71 to 1.44) | NR | NR |
ED: emergency department; HRQoL: health-related quality of life; HR: hazard ratio; NR: not reported; NA: not applicable; OR: odds ratio; RR: relative risk; SMD: standard mean difference; WMD: weighted mean difference.
aPrimary outcomes of QoL, hospitalization, ED visits, unscheduled/urgent health care use and mortality are reported. All outcomes are presented in detail in Table S1. When summarizing results, we included the longest term results reported. In terms of QoL outcomes across varying scales, we have included the total results (e.g. SGRQ total). All outcomes are presented in detail in Supplementary Table S1.
bTurnock et al. and Walters et al. both included pooled estimates for hospitalizations, but these were not presented as relative risks. Neither found a statistically significant impact. Walters also reported a pooled estimate for ED visits (no significant impact), but no estimate of relative risk.
cSt. George’s Respiratory Questionnaire (SGRQ) for QoL at >12 months.
dSGRQ total for HRQoL, results for SGRQ symptoms, impact, activity and CRQ reported in Supplementary Table S1.
Table 6.
Outcome: Quality of life, hospitalization and ED visits − significance of results per Table 2.
| Number of studies; participants | Systematic reviewa | Primary studiesb | Quality of life | Quality of evidence | Hospitalization | ED visits | Quality of evidence |
|---|---|---|---|---|---|---|---|
| Education/action plans | |||||||
| 13; 2239 (Effing) | High/High | High/High | + | Limited | ++ | NR | Strong |
| 12; 2103 (Tan) | High/High | High/High | + | +++ | NR | ||
| 3; 367 (Turnock) | High/Low | High/High | 0 | NR | NR | NA | |
| 5; 574 (Walters) | High/Low | High/High | 0 | NR | NR | ||
| Complex interventions with an SM focus | |||||||
| 32; 3941 (Dickens) | High/High | High/Med. | NR | NA | NR | +++ | Moderate |
| 26; 2997(Kruis) | High/High | High/High | 0 | Limited/moderate | 0 | 0 | |
| 29; 3688 (Zwerink) | High/High | High/High | +++ | +++ | NR | ||
| 10; 1502 (Jordan) | High/High | High/High | 0 | ++ | Not combined | ||
| Pulmonary rehabilitation | |||||||
| 65; 3822 (McCarthy) | High/High | High/High | +++ | Strong | NR | NR | NA |
| Telehealth | |||||||
| 10; 587 (Cruz) | High/Low | High/High | + | Limited | + | 0 | Moderate/strong |
| 9; 550 (Kamei) | Low/Low | Low/Med. | NR | +c | +++ | ||
| 10; 1004 (McLean) | High/High | High/High | 0 | +++ | ++ | ||
| Outreach nursing programme | |||||||
| 9; 1498 (Wong) | High/High | High/High | + | Limited | 0 | NR | Limited |
ED: emergency department; NR: not reported; NA: not applicable; Med.: medium; SM: self-management; COPD: chronic obstructive pulmonary disease
aSummary of systematic review quality based on R-AMSTAR score and number of participants, see Table 6.
bSummary of the quality of the primary studies based on the risk of bias and meta-analysis quality, see Table 6.
cResult for all COPD patients included, ++ for patients with severe and very severe COPD.
Quality appraisal and risk of bias
Systematic review quality, RCT and participant numbers, meta-analysis quality and the risk of bias of the underlying RCTs are included in Table 3. Three reviews were rated lower quality16,18,24 (R-AMSTAR <31) with the remainder rated higher quality. Six reviews had fewer than 1000 participants.14–16,23,24,28 The systematic reviews and meta-analyses are of high quality, but the underpinning RCTs are generally at high risk of bias. Results from the meta-analyses for the outcomes of HRQoL, healthcare utilization and mortality are discussed in the following summary of findings section and are summarized in Table 5.
Summary of findings
Education and action plans
Four reviews of 33 RCTs (26 unique RCTs) were identified, all from the PRISMS review. One Cochrane review (Effing et al.) assessed education with or without action plans.12 Education focused on improving COPD knowledge and understanding directed towards, for example, smoking cessation, improving exercise, inhalation techniques or coping strategies. A second Cochrane review assessed action plans not including education,14 defined as the use of guidelines outlining self-initiated interventions such as altering medication or visiting the hospital. The third review, an update of the previous Cochrane review assessing action plans but also included brief patient education.15 The fourth assessed patient managed disease-specific education programmes consisting of, for example, COPD information, smoking cessation suggestions, inhaler techniques, early recognition of signs requiring medical interventions and education.13 The author suggests that these programmes could be an alternative to time-consuming, expensive PR programmes.
All four reviews reported HRQoL outcomes with evidence rated as limited, two focused mainly on education showing a small but significant positive effect (Table 6).12,13 All four reported on hospitalization with evidence rated as strong, two mainly focused on action plans found no statistically significant impact14,15 and two focused on education showed a significant reduction.12,13 No reviews reported results for mortality. The fact that no further studies were retrieved in our updated search may indicate that it is clear that education forms an important part of SM but that further interventions to enhance this may improve results. This is evident from the review by Effing et al.,12 which was updated in 2014 by Zwerink et al.20 They noted that although patient education is an indispensable component of SM, education alone is insufficient to achieve the goal of behavioural change and to avoid ambiguity, they removed the term ‘education’ from the title of the review and broadened their inclusion criteria (i.e. no longer just education and action plans). As such, this review by Zwerink et al. is discussed in the section on ‘complex SM interventions’. This is in agreement with a recent international consensus statement on SM in COPD states that formalized education is centre stage to SM but while COPD SM interventions often include education and action plans, the intervention is considered to be more than the sum of these two components.4
Complex interventions with a focus on SM
Reviews of complex interventions with an SM focus are defined as those that typically included a range of SM interventions. They may involve multiple components and/or multiple professionals with the intervention delivered by a variety of means. Four reviews of 100 RCTs (77 unique RCTs) were included all of which were from our updated search.
One review assessed complex SM interventions as described earlier; these could use technology or include education or rehabilitation.17 The second review assessed integrated disease management interventions defined as programmes provided by caregivers from at least two different disciplines, with two different components (e.g., self-management, exercise, education).19 The third review assessed interventions with at least two interactions with a participant and healthcare provider and ideally also included formulation of goals and provision of feedback.20 It also had to include at least two of the following: smoking cessation, self-recognition and self-treatment of exacerbations, an exercise component, diet advice, medication advice or coping with breathlessness. Content could be delivered to study participants verbally, as written material or via audiovisual media. The fourth review assessed SM for patients with moderate to severe COPD, which included an SM component or package delivered to patients shortly after being discharged from hospital with a COPD exacerbation.21
Three reviews reported on HRQoL with evidence rated as limited to moderate,19–21 one review showed a significant positive effect20 (Table 5). Three reviews reported on hospitalization with evidence rated as moderate, two showed a significant reduction.20,21 One out of three reviews reported a significant reduction in emergency department (ED) visits.17 Three reviews reported on mortality with none showing a significant effect.19–21
Pulmonary rehabilitation
Although PR could be considered a complex intervention with a focus on SM, it is presented separately as a unique COPD SM intervention. We identified one review of 65 RCTs, a Cochrane review published in 2015 retrieved in our updated search.22
PR was defined as exercise training for at least 4 weeks with or without education and/or psychological support delivered to patients with exercise limitation attributable to COPD. This review reported on a primary outcome of HRQoL and a secondary outcome of exercise testing. Eleven out of 11 outcomes (eight for HRQoL and three for exercise capacity) showed a significant positive effect (Supplementary Table S1). The evidence for HRQoL was rated as strong. The authors report that large variation in the design of PR programmes makes it difficult to identify their optimal format.
Telehealth
Telehealth, telemedicine or telemonitoring are broad terms with no one universally accepted definition for each. A convention is developing, which uses ‘telehealth’ for health-based IT-based care, so we will use this term. Four reviews of 38 RCTs (30 unique RCTs) are included from our updated search.
One review assessed home telemonitoring for COPD patients where healthcare providers review patients’ clinical data more regularly and promptly to detect health deterioration.23 The second review assessed healthcare at a distance, involving communication of data between patient and health carer with feedback regarding COPD management.26 The third review included nurses monitoring patients from a telemonitoring centre and documenting their physical and mental status daily to allow for identification of early exacerbations and to provide education and mentoring from a distance.24 The fourth review assessed home-based solutions to making PR more accessible.28
Two of four included reviews reported HRQoL outcomes with evidence rated as limited. One review showed a positive effect for home telemonitoring23 (Table 6). Three of four reviews reported on hospitalization, with evidence rated as moderate to strong, all three showed a significant reduction23,24,26 with healthcare at a distance being the most significant.26 Three reviews reported on ED visits with two showing a significant positive effect.24,26 Two reviews reported on mortality, neither showed a significant effect.23,24
Outreach nursing
One review did not fit into the aforementioned categories and is more about the personnel delivering the support which was retrieved by PRISMS. It assessed an outreach nursing programme defined as home visits from a respiratory nurse or similar respiratory health worker to help people use their treatments well, provide education about coping strategies and disease monitoring.27 A small but significant improvement in HRQoL and no significant reductions in hospitalizations were reported based on evidence rated as limited.
Discussion
Summary of main findings
Reviews reporting on HRQoL were mainly positive with 6 out of 11 showing improvements. The strongest evidence was retrieved for PR, which reported significant improvements, followed by complex interventions with an SM focus based on evidence rated as limited to moderate. However, PR contains many components and it was not clear which components led to this positive result. Reviews that reported results for healthcare utilization were also mainly positive with seven out of eight reviews reporting positive results for hospitalization and three out of five reporting improvements for ED visits. The strongest evidence was retrieved for education followed by moderate to strong evidence for telehealth interventions and moderate evidence for complex interventions with an SM focus. There is no evidence that SM interventions have any effect on mortality.
Interpretation in relation to other literature
This overview was an update of an existing high-quality systematic overview (PRISMS). The PRISMS study included 14 long-term conditions, paediatric as well as adult populations, conducted a review of qualitative data and an implementation review. Specifically, PRISMS did not include telehealth-based interventions as they deemed them to be about the mode of delivery rather than the content of what was delivered. Telehealth is constantly evolving to incorporate new advancements and respond and adapt to changing health needs. As such, telehealth interventions were included in our updated review. While it was recognized that given the rate of technological advance, older telehealth reviews might be out of date, we were concerned that by simply updating the PRISMS study we would potentially miss out on pre-2013 reviews on this topic. As previously noted, our search was undertaken as part of a wider study that included reviews of SM interventions for other chronic diseases, a start date of 2009 and a wider search string. One additional high-quality COPD telehealth Cochrane review was identified and included to reduce the impact of this limitation. Overall, reviews retrieved, which assessed telehealth interventions, varied widely and in some cases only formed part of a more complex intervention making it difficult to draw conclusions.
Based on two reviews, PRISMS reported that education reduces hospitalization in COPD patients. We agree with this finding as we did not find any additional evidence for this intervention. The very fact that there are no further reviews of education may indicate that it is clear that education forms an important part of SM but that further interventions to enhance this may improve results. Effing et al.’s International consensus document states that formalized education is centre stage but while COPD SM interventions often include education and action plans, the intervention is considered to be more than the sum of these two components.4 We also retrieved additional reviews providing moderate to strong and moderate evidence of a reduction in healthcare utilization (hospitalization or ED visits) for telehealth interventions and complex interventions with an SM focus, respectively. PRISMS reported that consistent and less clinically significant effects were found for education in terms of HRQoL improvements across reviews. We found one additional review that did not alter this finding. However, we found strong evidence of significant improvements in HRQoL for PR. We also found limited to moderate evidence of improvements in HRQoL for complex interventions with an SM focus and limited evidence for telehealth interventions and outreach nursing programmes.
As with all overviews of reviews and systematic reviews, they are conducted for a specific point in time for which we can state where the evidence is at. There may have been reviews and RCTs published after this point in time, for example, Jonkman et al. published an individual patient data meta-analysis in 2016 (outside of our search dates), which aimed to identify which characteristics of COPD SM interventions are most effective and included 14 RCTs, 11 of which are included in this overview of reviews.29 The RCTs varied by intervention type (group sessions, individual sessions, exercise sessions, etc.) and duration. With respect to duration, some interventions included sessions over a longer period of time or included follow-up calls and were individual sessions on 1 day. Duration ranged from 1 day to (n = 1) to 24 months (n = 1) with an average duration of approximately 1 week. After adjusting for programme characteristics, they reported that longer duration SM interventions led to a reduction on all-cause hospitalizations in COPD patients. However, they didn’t specify if this should be in the form of follow-up calls or more sessions and they were unable to determine what the minimum required duration should be.
Future research and implications for decision makers
The clinical effectiveness of COPD SM interventions provides a complex picture. RCTs typically had small sample sizes and a short-term follow-up, limiting the applicability and validity of findings and potentially failing to capture long-term benefits. Although SM interventions are generally inexpensive on a per patient basis, the budget impact of these could be substantial due to large eligible patients’ numbers. It is important that the implementation and delivery of the interventions are subject to routine and ongoing evaluation. This would help ensure that they are delivering benefits to patients and allow the intervention content and format to be refined.
Almost all individuals eligible for COPD SM interventions will have more than one chronic disease that may also be suitable for SM.30 In these cases, consideration should be given to whether one disease-specific intervention is best and whether exposure to more interventions is overly burdensome. As such, implementation would require an evidence-based approach that acknowledges multimorbidity and considers how the main diseases interact.
Strengths and limitations
Overviews of reviews are gaining in popularity, an advantage being that they efficiently and systematically retrieve and summarize results of multiple systematic reviews into one report. A disadvantage is that they may not reflect the most recent literature as recent RCTs must first be captured in a systematic review to be included. However, given typically small sample sizes, it may not be appropriate to draw conclusions on the effect of an intervention based on a single or a number of small RCTs. Therefore, it is unlikely that more recent RCTs not captured in this overview of reviews would be sufficient to substantially alter recommendations informing major policy decisions. In addition, some reviews may include older RCTs with broader inclusion criteria which may distort results. We included two older Cochrane reviews but also included their updated versions which should minimize distortion of results.
Difficulties arise in distilling large bodies of literature into clear and useful findings hindered by the wide range of SM definitions in the literature; large range of SM interventions available; varying delivery formats and that intervention, for example PR, content can vary from programme to programme. Combining a large body of literature that assesses such a range of interventions is challenging. We broadly categorized our results into common SM interventions retrieved in our search to aid presentation of results. However, this broad categorization may be a limitation as there is still crossover between categories. To aid interpretation, we categorized evidence into ‘strong’, ‘moderate’ and ‘limited’ based on the quality of the evidence and the statistical significance of results (Table 2). These criteria are broadly based on the GRADE requirements,31 full GRADE criteria could not be adhered to as it is for grading primary RCTs included in systematic reviews. Our approach helps distil the information, but should not be reviewed in isolation from the detailed results to avoid misinterpretation. Issues may also arise conducting an overview of reviews as the reviewer is one step away from the primary evidence. We found that the systematic reviews and meta-analyses included were of high quality, but the underpinning RCTs were generally at high risk of bias. By assessing both the quality of the systematic review and meta-analysis and the risk of bias of the primary studies, we reduced the impact of this limitation. Finally, the potential for double counting of primary evidence or included RCTs was dealt with by assessing RCT crossover to ensure positive or negative results were not overemphasized if there was large overlap.
The Cochrane handbook provides valuable information on the overarching methods for overviews of reviews.32 However, more detailed guidance is required. A recent protocol published in 2016 aims to produce an evidence map of studies evaluating methods for conducting, interpreting and reporting overview of reviews, which should help standardize and optimize approaches.33
Gaps in the evidence
There is a lot of evidence for chronic disease SM. A factor that may contribute to inconsistent evidence is the lack of a clear SM definition across both primary studies and systematic reviews, which has changed over time. The inclusion and exclusion criteria of identified systematic reviews were often based on very broad descriptions of interventions, adding to the heterogeneity of data and may have introduced some distortion in the results. A consensus on the definition of SM in COPD by Effing et al. in 20164 is an important step forward and should ultimately help with future reviews of effectiveness of SM interventions in COPD by facilitating the identification of a more narrowly defined, but possibly less heterogeneous evidence-base; the proposed PRISMS taxonomy may help with this also.3 However, this agreed definition or taxonomy will still need to be fully and uniformly adopted and used for a time before its value can be realized. Any future reviews can be compared to this overview of reviews to determine whether the introduction of an agreed definition of COPD SM improves the review of effectiveness of these interventions providing recommendations for consensus in other chronic diseases.
Conclusion
These findings from a large body of evidence suggesting that SM, through education or potentially as a component of PR, confers significant health gains in people with COPD in terms of HRQoL. SM supported by telehealth confers significant reductions in health care utilization, including hospitalization and ED visits.
Supplementary Material
Acknowledgement
The authors would like to acknowledge the extended Health Information and Quality Authority (HIQA) evaluation team, the Expert Advisory Group (see www.hiqa.ie) and the PRISMS research assistants (E Epiphaniou, G Pearce and HL Parke).
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Supplemental material: Supplementary material is available for this article online.
References
- 1. World Health Organization. Chronic obstructive pulmonary disease (COPD). Fact sheet Nø315. Website 2015. Geneva: World Health Organization. [Google Scholar]
- 2. Adams K, Greiner AC, Corrigan JM. Report of a summit: the 1st Annual Crossing the Quality Chasm Summit: a focus on communities. National Academies Press; p. 57 Website 2004. [PubMed] [Google Scholar]
- 3. Pearce G, Parke HL, Pinnock H, et al. The PRISMS taxonomy of self-management support: derivation of a novel taxonomy and initial testing of its utility. J Health Serv Res Policy 2016; 21: 73–82. [DOI] [PubMed] [Google Scholar]
- 4. Effing TW, Vercoulen JH, Bourbeau J, et al. Definition of a COPD self-management intervention: International Expert Group consensus. Eur Respir J 2016; 48: 46–54. [DOI] [PubMed] [Google Scholar]
- 5. Taylor SJC, Pinnock H, Epiphaniou E, et al. A rapid synthesis of the evidence on interventions supporting self-management for people with long-term conditions: PRISMS - Practical systematic Review of Self-Management Support for long-term conditions. Health Serv Deliv Res 2014; 2: 173–188. [PubMed] [Google Scholar]
- 6. Higgins JPT, Green S. (eds). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011) The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org.
- 7. Higgins JP, Lane PW, Anagnostelis B, et al. A tool to assess the quality of a meta-analysis. Res Synth Methods 2013; 4: 351–366. [DOI] [PubMed] [Google Scholar]
- 8. Smith V, Devane D, Begley CM, et al. Methodology in conducting a systematic review of systematic reviews of healthcare interventions. BMC Med Res Methodol 2011; 11: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009; 151: 264–9, W64. [DOI] [PubMed] [Google Scholar]
- 10. Spruit MA, Singh SJ, Garvey C, et al. An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med 2013;188: e13–e64. [DOI] [PubMed] [Google Scholar]
- 11. Kung J, Chiappelli F, Cajulis OO, et al. From systematic reviews to clinical recommendations for evidence-based health care: validation of revised assessment of multiple systematic reviews (R-AMSTAR) for grading of clinical relevance. Open Dent J 2010; 4: 84–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Effing TW, Monninkhof EM, Van DV, et al. Self-management education for patients with chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2007; (3): CD002990. [DOI] [PubMed] [Google Scholar]
- 13. Tan JY, Chen JX, Liu XL, et al. A meta-analysis on the impact of disease-specific education programs on health outcomes for patients with chronic obstructive pulmonary disease. Geriatr Nurs 2012; 33: 280–296. [DOI] [PubMed] [Google Scholar]
- 14. Turnock AC, Walters EH, Walters JA, et al. Action plans for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2005; (4): CD005074. [DOI] [PubMed] [Google Scholar]
- 15. Walters JA, Turnock AC, Walters EH, et al. Action plans with limited patient education only for exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2010: (5): CD005074. [DOI] [PubMed] [Google Scholar]
- 16. Bentsen SB, Langeland E, Holm AL. Evaluation of self-management interventions for chronic obstructive pulmonary disease. J Nurs Manage 2012; 20: 802–813. [DOI] [PubMed] [Google Scholar]
- 17. Dickens C, Katon W, Blakemore A, et al. Complex interventions that reduce urgent care use in COPD: a systematic review with meta-regression. Respir Med 2014; 108: 426–437. [DOI] [PubMed] [Google Scholar]
- 18. Harrison SL, Janaudis-Ferreira T, Brooks D, et al. Self-management following an acute exacerbation of COPD: a systematic review. Chest 2015; 147: 646–661. [DOI] [PubMed] [Google Scholar]
- 19. Kruis AL, Smidt N, Assendelft WJ, et al. Integrated disease management interventions for patients with chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2013; 10: CD009437. [DOI] [PubMed] [Google Scholar]
- 20. Zwerink M, Brusse KM, van der Valk Paul D, et al. Self management for patients with chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2014; (3): CD002990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jordan RE, Majothi S, Heneghan NR, et al. Supported self-management for patients with moderate to severe chronic obstructive pulmonary disease (COPD): an evidence synthesis and economic analysis. Health Technol Assess 2015; 19: 1–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. McCarthy B, Casey D, Devane D, et al. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2015; 2: CD003793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cruz J, Brooks D, Marques A. Home telemonitoring effectiveness in COPD: a systematic review. Int J Clin Pract 2014; 68: 369–378. [DOI] [PubMed] [Google Scholar]
- 24. Kamei T, Yamamoto Y, Kajii F, et al. Systematic review and meta-analysis of studies involving telehome monitoring-based telenursing for patients with chronic obstructive pulmonary disease. Jpn J Nurs Sci 2013; 10: 180–192. [DOI] [PubMed] [Google Scholar]
- 25. Lundell S, Holmner A, Rehn B, et al. Telehealthcare for patients with COPD, effects on physical activity level, physical capacity and dyspnea: A systematic review and meta-analysis. Eur Respir J 2014; 44: 1–8.24982045 [Google Scholar]
- 26. McLean S, Nurmatov U, Liu JL, et al. Telehealthcare for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2011; (7): CD007718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wong CX, Carson KV, Smith BJ. Home care by outreach nursing for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2011; 4: CD000994. [DOI] [PubMed] [Google Scholar]
- 28. Lundell S, Holmner A, Rehn B, et al. Telehealthcare in COPD: a systematic review and meta-analysis on physical outcomes and dyspnea. Respir Med 2015; 109: 11–26. [DOI] [PubMed] [Google Scholar]
- 29. Jonkman NH, Westland H, Trappenburg JC, et al. Characteristics of effective self-management interventions in patients with COPD: individual patient data meta-analysis. Eur Respir J 2016; 48: 55–68. [DOI] [PubMed] [Google Scholar]
- 30. Barnett K, Mercer SW, Norbury M, et al. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. Lancet 2012; 380: 37–43. [DOI] [PubMed] [Google Scholar]
- 31. Guyatt G, Oxman AD, Akl EA, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol 2011; 64: 383–394. [DOI] [PubMed] [Google Scholar]
- 32. Becker LA, Oxman AD. Chapter 22: Overviews of reviews In: Higgins JPT, Green S. (eds), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org
- 33. Lunny C, Brennan SE, McDonald S, et al. Evidence map of studies evaluating methods for conducting, interpreting and reporting overviews of systematic reviews of interventions: rationale and design. Syst Rev 2016; 5: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.