Description
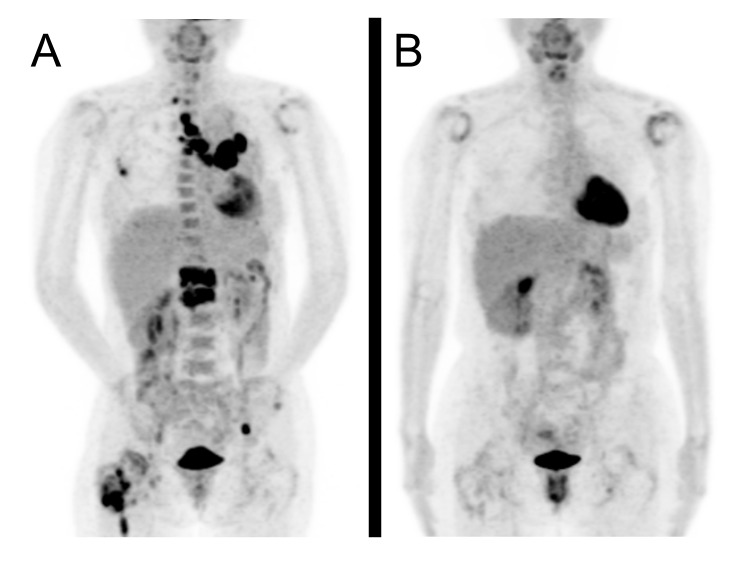
A 48-year-old Japanese woman with non-small cell lung adenocarcinoma (cT3N2M1b) received nivolumab (an immune checkpoint inhibitor) at a dosage of 3 mg/kg every 2 weeks. Treatment with nivolumab was effective. The primary tumour as well as metastases to the lymph nodes and spine almost completely disappeared, as shown on positron emission tomography-CT before treatment (figure 1A) and 4 months after treatment (figure 1B). However, she developed sustained pain and swelling in the shoulders and knees bilaterally after only one infusion of nivolumab, and active inflammation was detected in the shoulder joints bilaterally (figure 1B). She had no personal and family histories of autoimmune disease. Serum C-reactive protein level was 2.1 mg/L. Serum matrix metalloproteinase-3 was elevated at 209 ng/mL (normal <60). Antinuclear antibody, rheumatoid factor and anti-cyclic citrullinated peptide antibody were negative. Ultrasonography confirmed shoulder tenosynovitis and bursitis (figure 2). The arthritis responded well to low-dose prednisone, and she could continue to receive nivolumab. General physicians are encountering an increasing number of immune-related adverse events with increased use of immune checkpoint inhibitors.1 Our case provides instructive images demonstrating both the efficacy of immune checkpoint inhibitors and the associated immune-related adverse events.
Figure 1.
Positron emission tomography-CT before treatment (A) and 4 months after treatment (B) with nivolumab. The primary tumour as well as metastases to the lymph nodes and spine seen before treatment almost completely disappeared. On the other hand, active inflammation was detected in the shoulder joints bilaterally (B).
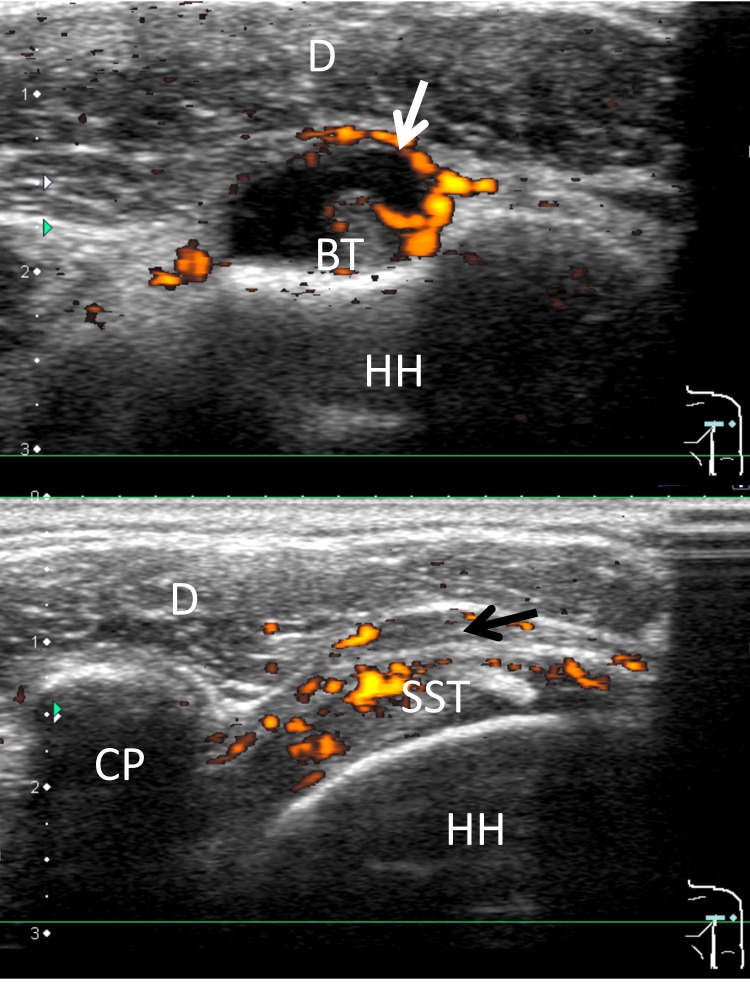
Figure 2.
Ultrasonography showing shoulder tenosynovitis (white arrow) and bursitis (black arrow). BT, biceps tendon; CP, coracoid process; D, deltoid muscle; HH, humeral head; SST, supraspinatus tendon.
Learning points.
An increasing number of immune-related adverse events have been described with the increased use of nivolumab, an immune checkpoint inhibitor.
This case provides instructive images showing both the efficacy of the immune checkpoint inhibitors and the associated immune-related adverse events.
Footnotes
Contributors: SK and YM involved in conception or design of the work. SK and KK are responsible for acquisition of data. SK, KK, TM and YM are responsible for analysis and interpretation of data. SK, TM and YM drafted or revised the manuscript.
Competing interests: SK, TM and YM received scholarship donations from AbbVie, Actelion, Astellas, Bristol-Myers, Chugai, Daiichi-Sankyo, Eisai, Eli Lilly, Japan Blood Products Organization, Mitsubishi-Tanabe, MSD, Pfizer, Shionogi, Takeda, Teijin and UCB. KK received scholarship donations from Astellas, Boehringer lngelheim, Chugai, MSD, Ono and Taiho.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
Reference
- 1.van der Vlist M, Kuball J, Radstake TR, et al. . Immune checkpoints and rheumatic diseases: what can cancer immunotherapy teach us? Nat Rev Rheumatol 2016;12:593–604. doi:10.1038/nrrheum.2016.131 [DOI] [PubMed] [Google Scholar]