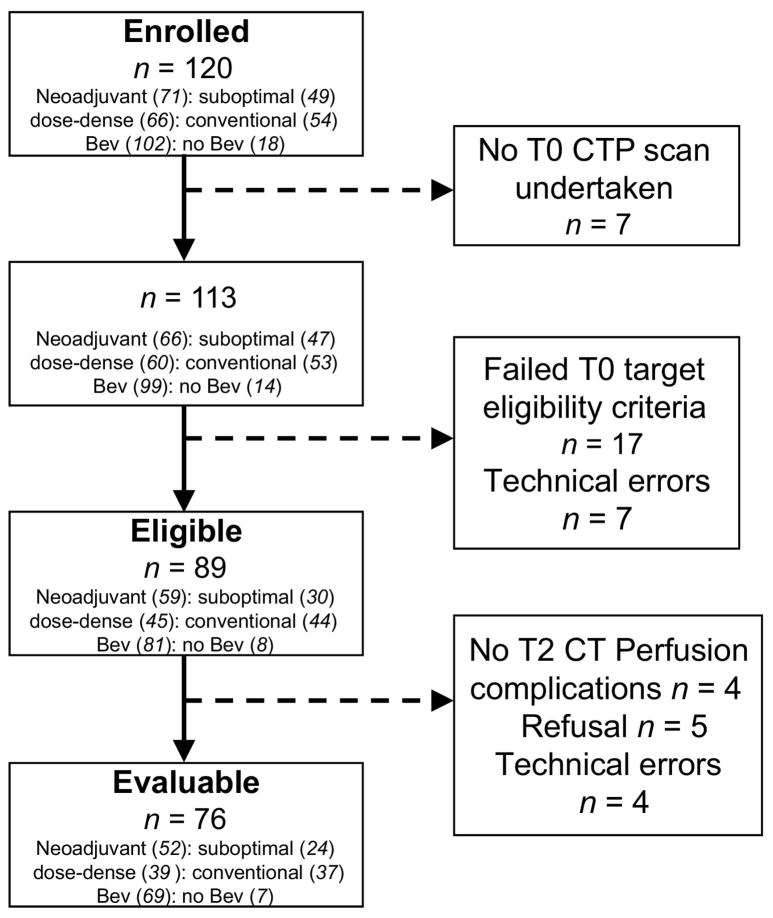
Figure 1.
Flow diagram of patient recruitment in ACRIN 6695. Eligible: target lesion met target lesion criteria on central review; Evaluable: patient with technically analyzable CTP scans of the same target lesion from T0 and T2; Suboptimal: Suboptimally debulked tumor; Dose-dense: weekly paclitaxel; Conventional: 3-weekly paclitaxel; Bev =Bevacizumab