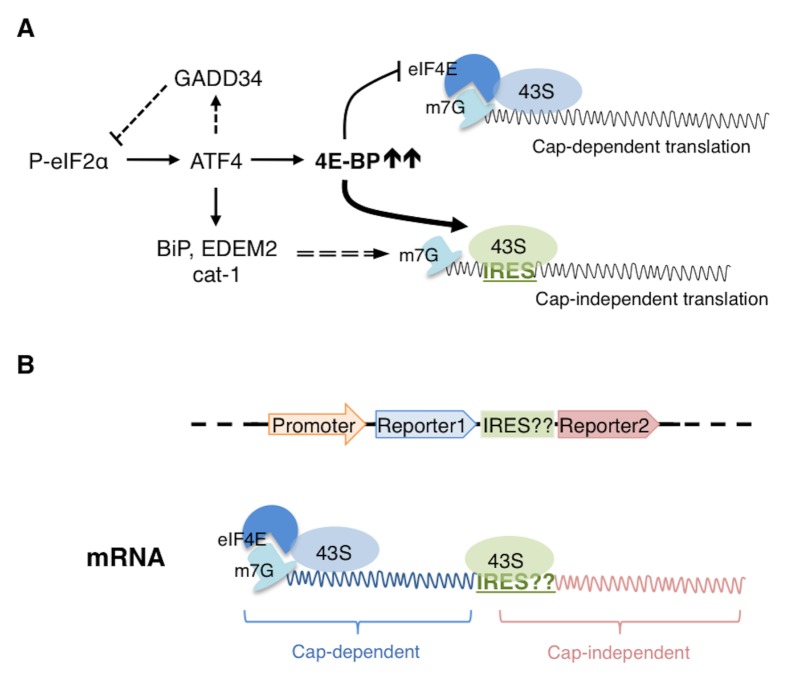
Fig. 3.
4E-BP mediated Cap-independent translation. (A) Translation attenuation by phospho-eIF2α is relieved by a feedback loop involving an eIF2α phosphatase regulatory subunit, GADD34. However, further translation inhibition is imposed by 4E-BP, an ATF4 target. 4E-BP sequesters eIF4E, which is the m7G caprecognition protein. Cap recognition by eIF4E is required for the efficient recruitment of the 43S subunit and thus in the presence of 4E-BP, cap-dependent translation is negatively affected. Under such conditions however, transcripts with IRESes in their 5′UTRs are able to recruit 43S independent of cap-recognition and are thus translated in cap-independent manner. The 5′UTRs of several stress response transcripts, including BiP, EDEM2 and cat-1 have been shown to have IRES elements. (B) The schematic on top shows the arrangement of the elements of a bicistronic construct to test the potential IRES activity of a given 5′UTR (indicated as ‘IRES??’). Expression of this construct in cells results in an mRNA that can recruit 43S in a cap-dependent way, leading to translation of reporter1. If the given 5′UTR has IRES activity, then it can independently recruit 43S for the translation of reporter2. Thus, if expression of both reporters were detected, it would indicate that the given 5′UTR likely has IRES activity.