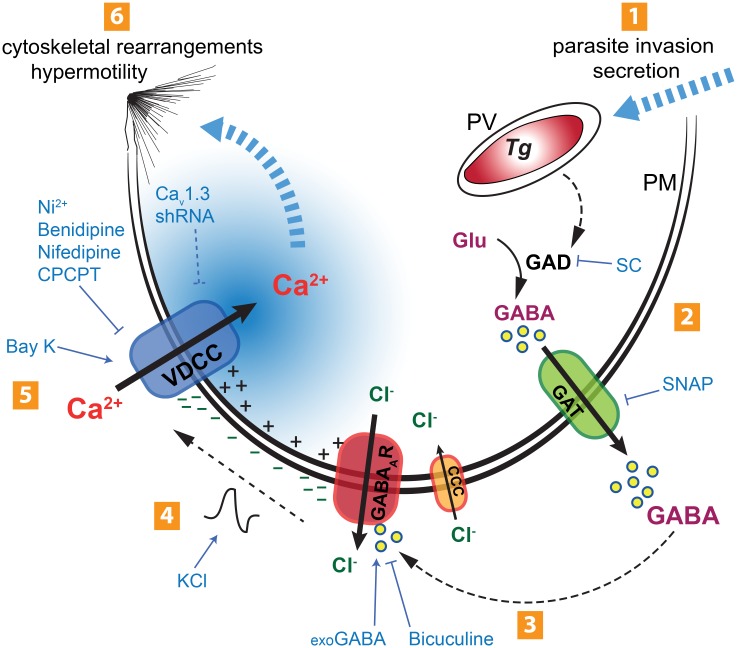
Fig 9. Schematic representation of the proposed mechanism for the initiation of the hypermigratory phenotype in Toxoplasma-infected DCs.
(1) Active invasion by T. gondii (Tg) of the host cell across its plasma membrane (PM) involves secretory processes. Inside the host cell, T. gondii resides in the parasitophorous vacuole (PV). (2) Parasite invasion sparks an increase in GABA synthesis by host-cell glutamate decarboxylase (GAD) that can be inhibited by semicarbazide (SC) [8]. Synthesized GABA is secreted through GABA transporters (GAT). Inhibition of GABA synthesis (SC) or GABA secretion (SNAP) abolishes DC hypermotility [8]. (3) GABA activates GABAA receptor (GABAAR) channels on the host cell surface by an autocrine loop, leading to chloride (Cl-) efflux. The GABAAR antagonist bicuculine inhibits hypermotility and exogenous GABA (exoGABA) reconstitutes hypermotility. Hypothetically, the Cl- gradient is maintained by cation-chloride co-transporters (CCC). (4) Efflux of Cl- leads to membrane depolarization (-). Induced membrane depolarization by potassium chloride (KCl) or GABA can also reconstitute hypermotility. (5) Voltage-dependent calcium channels (VDCCs; primarily Cav1.3) open in response to membrane depolarization, leading to Ca2+ influx. Blockade of membrane-bound calcium channels by nickel (Ni), inhibition of VDCCs by nifedipine or benidipine, specific inhibition of Cav1.3 (CPCPT) or ablation of CaV1.3 expression by shRNA (CaV1.3 shRNA) have inhibitory effects on hypermotility, while the VDCC agonist Bay K 8644 (Bay K) induces motility and reconstitutes hypermotility upon GABAergic inhibition. Upon Cav1.3 blockade, GABA cannot induce or reconstitute hypermotility. (6) Hypothetically, the Ca2+ transient generated may be propagated to intracellular Ca2+ channels and stores. The altered Ca2+ signaling pattern may activate the downstream migratory machinery and MAP kinase regulators (e.g. 14-3-3), leading to cytoskeletal rearrangements and shifting the cell into a hypermotile state.