Abstract
Introduction
Metformin use in advanced chronic kidney disease is controversial. This study sought to examine the pharmacokinetics, safety, and efficacy of low-dose metformin in patients with type 2 diabetes and stage 4 chronic kidney disease.
Methods
In this open-label, phase I trial, 3 consecutive cohorts (1, 2, and 3) of 6 patients each were recruited to receive 250-, 500-, or 1000-mg once-daily doses of metformin, respectively. All patients underwent a first-dose pharmacokinetic profile and weekly trough metformin concentrations for the duration of 4 weeks of daily therapy. Prespecified clinical and biochemical safety endpoints of serum bicarbonate, venous pH, and serum lactate were assessed weekly. Efficacy was assessed by pre- and post-HbA1c and 72-hour capillary glucose monitoring.
Results
There was no evidence of accumulation of metformin in any cohort. There were no episodes of hyperlactatemia or metabolic acidosis and no significant change in any biochemical safety measures. Median (interquartile range) observed trough concentrations of metformin in cohorts 1, 2, and 3 were 0.083 (0.121) mg/l, 0.239 (0.603) mg/l, and 1.930 (3.110) mg/l, respectively. Average capillary glucose concentrations and mean HbA1c decreased in all cohorts.
Discussion
In our patient cohorts with diabetes and stage 4 chronic kidney disease, treatment with 4 weeks of low-dose metformin was not associated with adverse safety outcomes and revealed stable pharmacokinetics. Our study supports the liberalization of metformin use in this population and supports the use of metformin assays for more individualized dosing.
Keywords: chronic kidney disease, diabetes mellitus, metformin, pharmacokinetics, phase I trial, safety
Type 2 diabetes is increasingly the most prevalent cause of chronic kidney disease (CKD) worldwide, with a lifetime prevalence of nephropathy of ∼40%.1 Metformin is a time-tested medication in the treatment of type 2 diabetes and is the recommended first-line drug in almost all practice guidelines.2, 3 Metformin is considered to have long-term beneficial effects on cardiovascular and all-cause mortality in patients with type 2 diabetes with normal and reduced renal function.4
Unfortunately, there has been a reluctance to use metformin in CKD due to concerns of drug accumulation leading to metformin-induced lactic acidosis. In recent years, however, there has been a trend toward liberalization of metformin use in CKD. In April 2016, the US Food and Drug Administration, while still following a threshold approach, dropped the threshold for discontinuing metformin to an estimated glomerular filtration rate (eGFR) of 30 ml/min per 1.73 m.2, 5 In New Zealand, it has been common practice to continue metformin at an unchanged dose until a patient passes an eGFR threshold of 30 ml/min per 1.73 m2 despite official guidance recommending a cutoff of 60 ml/min per 1.73 m2. However, in September 2015, the lower threshold was reduced from 60 to 15 ml/min per 1.73 m2 while mandating a graded dose reduction as the eGFR decreases.6
Despite the availability of cross-sectional studies, case reports, systematic reviews, and commentaries,7 there is a dearth of pharmacokinetic and pharmacodynamic studies of metformin in moderate to severe CKD. Kajbaf et al.8 recently observed that, even today, the question of metformin’s therapeutic range still remains uncertain. This paucity of pharmacokinetic data in the context of CKD may lead to hesitancy in prescribing.
In this paper, we report the pharmacokinetics, pharmacodynamics, toxicity, and inter- and intrapatient variability of low-dose metformin in patients with diabetes and stable stage 4 CKD (15 ms > eGFR < 30 ml/min per 1.73 m2). We used plasma metformin concentration as a surrogate safety marker, carefully monitoring for adverse events and biochemical signs of lactic acidosis over a treatment period of 4 weeks.
Subjects and Methods
This open-label, prospective, phase I, single-center (Middlemore Hospital, Auckland, New Zealand) safety study was conducted in a cohort of patients with type 2 diabetes and stable stage 4 CKD. Ethics approval was obtained from the New Zealand health and Disability Ethics Committees (reference number NTX/11/12/112). All patients provided written informed consent and the study was conducted between June 2012 and June 2014.
Inclusion/Exclusion Criteria
Patients were included if they were between 30 and 75 years of age, had a diagnosis of type 2 diabetes for at least 2 years, an HbA1c level between 6% and 11% (42 and 97 mmol/mol) and stage 4 CKD as defined by a stable eGFR between 15 and 30 ml/min per 1.73 m2 over the preceding 3 months. Exclusion criteria were a history of metformin intolerance, pregnancy, breastfeeding, existing metabolic acidosis, or having significant risk factors for metabolic acidosis. These included morbid obesity (>160 kg), unstable ischemic heart disease, a planned radiocontrast examination in the next 6 months or relevant medical comorbidities such as severe chronic obstructive pulmonary disease, unstable congestive heart failure, and significant liver disease.
Study Protocol
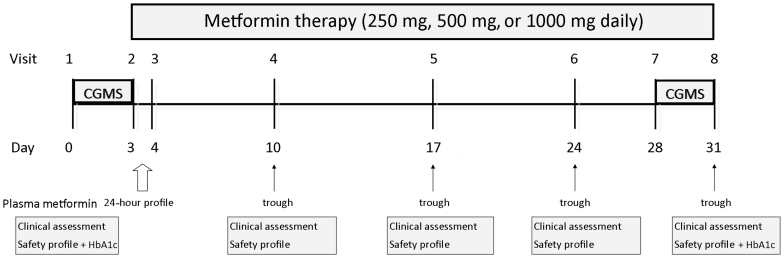
The study protocol is summarized in Figure 1. Three cohorts (6 patients each) underwent the trial sequentially with cohorts 1, 2, and 3 receiving metformin 250 mg, 500 mg, and 1000 mg of metformin, respectively, for 4 weeks. This was administered as a single daily morning dose before breakfast. Cohort 1 safety profile results were analyzed by an independent nephrologist before embarking on treatment of cohort 2. Cohort 3 treatment followed cohort 2, ensuring safety on similar lines.
Figure 1.
Study design. CGMS, 72-hour capillary glucose monitoring; Safety profile—serum lactate, bicarbonate, venous pH, renal function and electrolytes, liver enzymes, and full blood count.
Each patient was asked to attend our research facility on a Monday morning when a 72-hour continuous capillary glucose monitoring system (CGMS) was attached, and a fasting metabolic profile (glucose, insulin, lipids, and HbA1c) was performed. A safety profile using venous blood from the antecubital fossa constituting serum lactate, bicarbonate, venous pH, renal function and electrolytes, liver enzymes, and full blood count was also performed.
Visit 2 took place 3 days later (Thursday) when the CGMS was removed, and the first dose of metformin was taken. A pharmacokinetic profile was performed with measurements of plasma metformin concentrations at 0, 2, 4, 6, 8, and 24 hours.
The patients continued to take daily metformin and returned for weekly visits to assess for adverse events and measurement of trough metformin concentration and safety profile. The 72-hour CGMS was repeated in the last 3 days of metformin therapy (Monday through Thursday of week 4), with HbA1c measured at the end of the trial.
Metformin Assay
A high-performance liquid chromatography assay was used for the determination of metformin in plasma, as has been described and validated previously by Zhang et al.9
Outcomes
The primary safety outcome was the development of acidosis. This was assessed by measuring fasting levels of venous lactate, bicarbonate, and pH. A secondary safety endpoint was a trough concentration of metformin <5 mg/l.
As this was primarily a safety and not an efficacy study, the main pharmacokinetic parameters considered were repeat-dose trough concentrations, maximum concentration (Cmax), and time to maximum concentration (tmax) over the 24-hour period following the initial dose.
Efficacy was assessed by measuring HbA1c and CGMS at the beginning and end of 4 weeks of treatment.
Statistical Analysis
All statistical analyses were conducted using the R Environment for statistical computing. A P value of 0.05 was considered significant. Descriptive statistics were used to summarize baseline characteristics. Concentration-time curves were summarized using descriptive statistics computed over patients within time points. Areas under the concentration-time curves were estimated using a first-order compartment model with absorption.10
Further statistical modeling was undertaken to generate 95% prediction intervals (intervals within which future patient responses can be expected to lie with 95% confidence) for each safety variable. Linear mixed models with random intercepts for patients were fitted using all available data. All models included fixed-effects terms for the outcome at baseline, study day, and study cohort. Random intercepts for patients were included to account for correlation among repeated measurements taken on the same patient. The upper limits of the prediction intervals were compared with locally established safety thresholds for outcomes where values above the threshold are of concern (e.g., venous lactate), and the lower limits were compared with thresholds for outcomes where values below the threshold are of concern (e.g., pH, bicarbonate). The prediction intervals were a function of outcome values at baseline because these were included in the models. To effect a conservative approach for each outcome, the baseline value that yielded prediction intervals with upper/lower limits closest to the respective threshold was used to assess safety. We compared safety thresholds with the limits of prediction intervals from a statistical model, as opposed to observed values, to account for random variation.
Change in HbA1c from baseline was investigated using a linear mixed model with the change in HbA1c from baseline to last visit as the response, adjusting for baseline HbA1c and study cohort. The cohort with a 250-mg dose was the reference category. Metformin trough concentrations measured at the start of each visit were investigated using a linear mixed model with random intercepts for patient. Study day, study cohort, and BMI were included as fixed effects. Creatinine clearance and eGFR were also included so as to assess their association with trough concentrations. Metformin trough concentrations were log transformed to improve model fit but were not interpreted directly. Of primary interest were the regression model coefficients for creatinine clearance and eGFR to assess their association with trough concentrations. Accumulation was assessed using the coefficient for study day.
CGMS profiles were also investigated using linear mixed models. To account for missing values, the nonmissing values were scaled to give an average hourly concentration per patient per visit. Weights were used in the regression models to account for the differing numbers of hours of data across patients. Unadjusted and adjusted models for change in concentration from baseline were reported. The unadjusted model contained fixed-effects terms for the effect of follow-up, study cohort, and their interaction. The adjusted model included a fixed effect for baseline HbA1c.
Results
The study cohort consisted of 18 patients with type 2 diabetes mellitus and stable stage 4 CKD. Baseline characteristics of the participants are shown in Table 1. Median (interquartile range) age, body mass index, and duration of diabetes in all participants were 66.0 (6.54) years, 38.0 (9.87) kg/m2, and 15.0 (7.75) years, respectively. Cohort 1 had the largest mean body mass index (43.8; interquartile range, 5.0), and cohort 3 had the lowest (31.4; interquartile range, 7.1). Consistent with our service population, there was a high representation of Maori and Pacific people (N = 13) in our cohort, and there were more males (N = 14).
Table 1.
Baseline characteristics of the study population
| Characteristic | Group 1: 250 mg | Group 2: 500 mg | Group 3: 1000 mg |
|---|---|---|---|
| Age, yr | 64 (42–74) | 64.3 (49–70) | 67.8 (61–72) |
| Sex, M/F | 4/2 | 6/0 | 5/1 |
| Weight, kg | 118.4 (20.8) | 111.4 (22.9) | 85 (30.9) |
| BMI | 43.8 (5.0) | 37.1 (8.8) | 31.4 (7.1) |
| Diabetes duration, yr | 13.5 (5.5) | 14 (3.5) | 20 (8.0) |
| pH, mmol/l | 7.30 (0.02) | 7.28 (0.05) | 7.28 (0.05) |
| Lactate, mmol/l | 0.95 (0.25) | 1.35 (0.48) | 1.0 (0.58) |
| Bicarbonate, mmol/l | 26.5 (1.8) | 24.0 (3.5) | 22.5 (1.75) |
| eGFR, ml/min per 1.73 m2 | 21.0 (3.75) | 25.0 (10.3) | 19.5 (4.0) |
| Creatinine, μmol/l | 259.5 (63) | 244.5 (83.5) | 256.5 (58.3) |
| CrCl, ml/min | 28.7 (11.2) | 32.5 (17.5) | 27.5 (6.1) |
| CrCl using IBW, ml/min | 19.7 (8.7) | 25.7 (14.3) | 23.5 (5.3) |
| HbA1c, mmol/mol | 59.5 (15) | 69.5 (27) | 82.0 (17.3) |
| HbA1c, % | 7.6 (3.5) | 8.5 (4.6) | 9.7 (3.7) |
| Oral agents only | 2 | 2 | 1 |
| Insulin only | 2 | 0 | 2 |
| Insulin and oral agents | 2 | 4 | 3 |
BMI, body mass index; CrCl, creatinine clearance; eGFR, estimated glomerular filtration rate; IBW, ideal body weight; M/F, male/female.
Data are presented as number, median (IQR), or range for age.
The median baseline eGFR for all our participants was 21.0 (8.0) ml/min per 1.73 m2 (Modification of Diet in Renal Disease), an HbA1c level of 67.5 (interquartile range, 25.75) mmol/mol (8.3%; interquartile range, 4.5%), venous pH of 7.3 (interquartile range, 0.05), and serum lactate 1.05 (interquartile range, 0.58) mmol/l. The lowest baseline eGFR was 15 ml/min per 1.73 m2 in a patient in cohort 2; the lowest eGFR for a patient in cohort 3 was 17 ml/min per 1.73 m2.
Safety
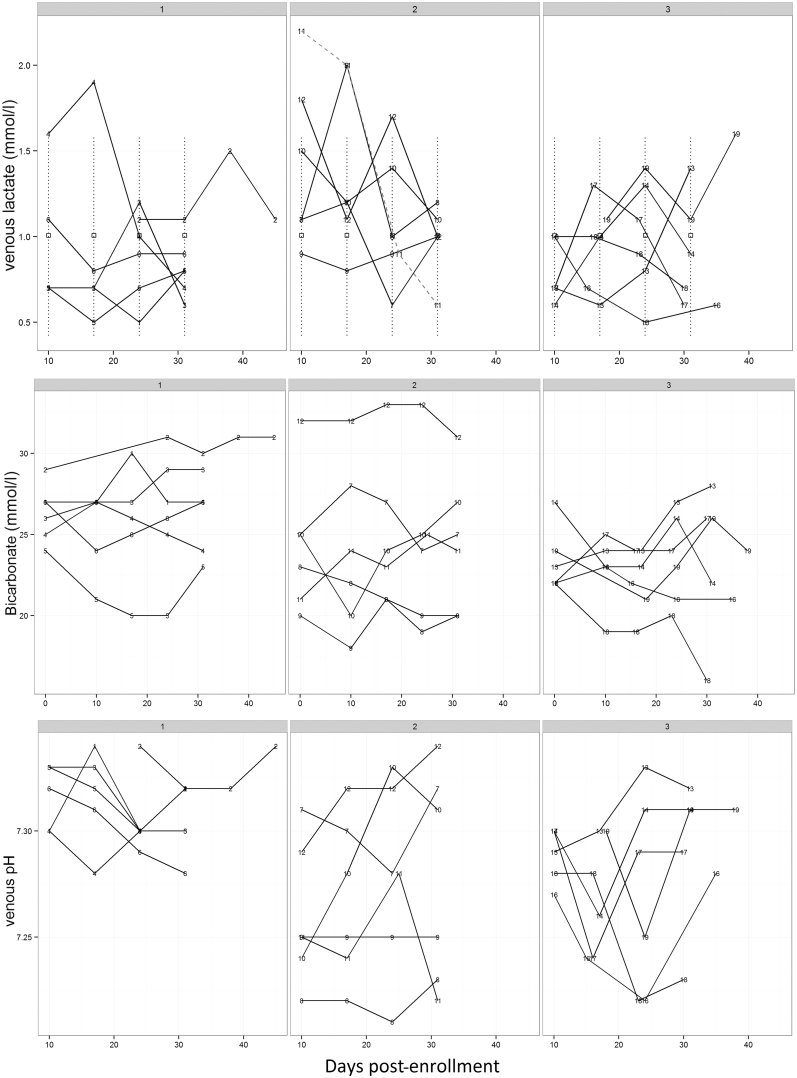
No adverse events were reported during the trial, and lactic or metabolic acidosis developed in none of our patients. Overall, there was no evidence of an effect of different metformin doses or treatment duration on lactate or bicarbonate levels (Figure 2).
Figure 2.
Safety profile (venous lactate, bicarbonate, and pH) in all 3 cohorts across the study period. Venous lactate for patient 11 appears as a dotted line. Patients 2 and 19 commenced metformin therapy a few days after enrollment in study.
Interestingly, relative to cohort 1, the venous pH throughout the trial period was 0.06 lower in cohort 2, and this was statistically significant (95% confidence interval −0.11 to −0.015); however, this was not apparent in cohort 3 (95% confidence interval −0.09 to 0.01). The lowest value of venous pH during treatment was in a patient in cohort 2 at 7.21 at 3 weeks (initial pH, 7.28; final pH, 7.23) with a lactate level of 1.0 mmol/l. The lowest observed baseline pH was 7.2. The lower limit of the prediction intervals for a patient with this baseline was 7.19.
The lowest value of bicarbonate (16 mmol/l) was found in 1 patient in cohort 3 at 30 days into treatment, but this was not associated with any change in lactate, pH, or eGFR. Prediction intervals were constructed using the lowest observed value of bicarbonate at baseline, which was 20 mmol/l. The lowest limit of the prediction intervals for a patient with this baseline bicarbonate was 15.7 mmol/l.
The highest level of lactate recorded was in a patient in cohort 2, which was 2.5 mmol/l. This was at baseline and decreased consistently at each visit while on therapy to 0.6 mmol/l at the last visit and was not associated with any change in venous pH or bicarbonate. The effect of baseline lactate on venous pH was not significant (P = 0.94). The largest of the upper limits of prediction intervals for a patient with a baseline venous lactate level of 2.5 mmol/l was 1.89 mmol/l. This is lower than the baseline used to derive the limits because observed lactate values post-baseline were all lower than 2.5 mmol/l.
Pharmacokinetic Profiles
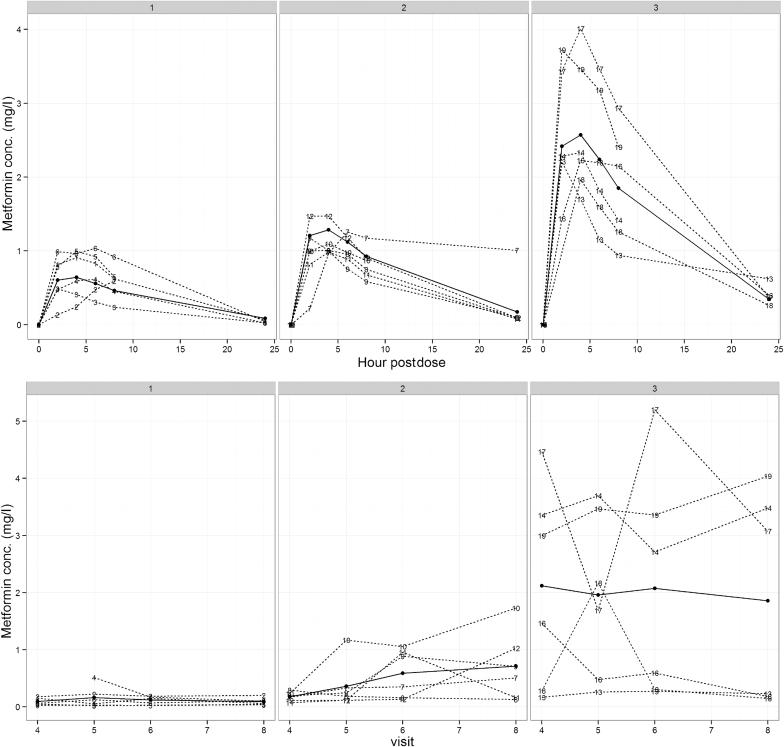
The first dose pharmacokinetic profiles and median pharmacokinetic parameters across the cohorts are presented in Figure 3 and Table 2, respectively.
Figure 3.
Pharmacokinetic profiles in all 3 cohorts across the study period. The first graph represents the 24-hour concentration-time curve after the first dose of metformin. The dotted lines are observed patient values. The solid line represents a fitted curve for a first-order compartment model with absorption in the peripheral compartment. The second graph represents all trough concentrations at a steady state over 4 weeks. The actual and mean values are presented as dotted and solid lines, respectively. Number labels represent patients in the trial. conc., concentration.
Table 2.
Pharmacokinetic profile across study groups
| Pharmacokinetic measure | Group 1: 250 mg | Group 2: 500 mg | Group 3: 1000 mg |
|---|---|---|---|
| Cmax, mg/l | 0.76 (0.38) | 1.13 (0.21) | 2.28 (1.16) |
| tmax, h | 5 (2.00) | 4 (0.00) | 4 (1.50) |
| AUC, mg.h/l | 8.43 ± 0.83 | 16.87 ± 1.66 | 33.74 ± 3.31 |
| Trough, mg/l | |||
| Week 1 | 0.062 (0.101) | 0.179 (0.995) | 2.23 (2.69) |
| Week 2 | 0.0885 (0.162) | 0.215 (0.174) | 1.930 (2.37) |
| Week 3 | 0.148 (0.103) | 0.616 (0.734) | 1.650 (2.82) |
| Week 4 | 0.0785 (0.056) | 0.607 (0.702) | 1.651 (3.19) |
| All weeks | 0.0830 (0.121) | 0.239 (0.603) | 1.930 (3.11) |
AUC, area under the concentration-time curve.
Data presented as median (IQR) or mean ± SD for the AUC.
In each cohort, there was no evidence of an increase in metformin levels after the first week of therapy, suggesting no accumulation of the drug during the study period (P = 0.17). Mean trough concentrations over the duration of the study for cohorts 2 and 3 were significantly greater than those for cohort 1 (P values of 0.02 and 0.0001, respectively.). Only 1 trough concentration was >5 mg/l (5.204 mg/l) in a patient in cohort 3, but this subsequently fell to 3.074 mg/l, and all safety parameters were unchanged in this patient (lactate 1.1 mmol/l).
There were a wide interpatient variability and inconsistent intrapatient variability in trough concentrations noticeable in cohort 3. Some of these variable readings seemed to be outliers from expected within patient variability, and we cannot exclude the possibility that patients might, on occasion, have taken their morning dose before the blood test (patient 11, week 3; patient 12, week 4; patient 16, week 1; patient 18, week 2). However, these were assumed as true troughs as compliance was not formally assessed and included in the data analysis. This did not appear to cause any safety concerns.
For the entire patient cohort, the predictive power of the eGFR and creatinine clearance, as measured by Cockroft-Gault formula on metformin concentrations, was almost identical (Spearman rank correlation of –0.215 and 0.287, respectively).
More details regarding pharmacokinetics profiles and modeling of metformin derived from this study will be published separately.
Efficacy
HbA1c decreased, on average, in all cohorts over the course of the trial. The mean (± SD) decreases in HbA1c from baseline to last visit were 3.00 ± 2.76, 2.33 ± 4.18, and 13.00 ± 6.78 mmol/mol for cohorts 1, 2, and 3, respectively. The average decrease for cohort 1 was significantly different from 0 (P = 0.001). The difference between average decreases for cohorts 1 and 2 was not significant (P = 0.20), but that between cohorts 1 and 3 was (P = 0.04).
The unadjusted estimated mean change in glucose in cohort 1 was −2.58 mmol/l (P = 0.01, 95% confidence interval −5.69 to 0.54). Mean changes were not different across cohorts (P values of 0.54 and 0.89). On adjusting for baseline HbA1c, the mean change in glucose was unchanged at −2.58 mmol/l (P = 0.06, 95% confidence interval −5.28 to 0.126). Again, cohort effects were not significant (P = 0.48 and 0.87). The effect of baseline HbA1c was significant (P = 0.002).
Discussion
To our knowledge, this is the largest prospective trial of metformin in patients with type 2 diabetes with stage 4 kidney disease to date. Our study suggests that metformin in these doses is safe, with no patients experiencing adverse effects. Lactic acidosis developed in none of our patients, and there was no reduction in serum bicarbonate or venous pH during treatment. Furthermore, over this limited trial period, there was a reduction in HbA1c and a reduction in mean glucose levels as measured by CGMS, which is consistent with a beneficial therapeutic effect even at lower doses.
Our results also demonstrate that metformin exhibits a predictable first-dose pharmacokinetic profile in stage 4 CKD consistent with results in subjects with preserved renal function. Our trough metformin concentrations do not indicate drug accumulation over the study period.
Trough values were consistent with those of other pharmacokinetic studies in patients with CKD 4.11, 12, 13, 14 In a trial of 24 patients with a creatinine clearance of 15 to 40 ml/min, with 135 measures of metformin concentration, Duong et al.13 did not find any episodes of lactic acidosis. This particular cohort also included 2 patients on dialysis. Peak plasma metformin concentration was always <5 mg/l. In a study by Frid et al.,14 nine patients with an eGFR <30 had metformin concentrations measured every 2 weeks over 8 weeks that showed a median trough level for metformin of 1.15 mg/l. In 35 patients on peritoneal dialysis taking 500 to 1000 mg/day examined by al Al-Hweish et al.,12 mean plasma metformin was 2.6 ± 1.5 mg/l, and none of the patients had episodes of lactic acidosis.
One notable aspect of our results was the variability in trough levels between patients. It has been reported that oral bioavailability and renal clearance of metformin is significantly variable between individuals.15 This might be especially clinically relevant in the context of advancing CKD. In cohort 3 of our study, patient 14, who had a baseline eGFR of 23 ml/min per 1.73 m2, had a mean metformin concentration of 3.310 mg/l, whereas patient 15 with an eGFR of only 19 ml/min per 1.73 m2 had a concentration of only 0.676 mg/l. These results raise the possibility that standard dosing based only on broad eGFR bands without reference to individual pharmacokinetics may lead to undertreatment or risk toxicity in some cases.
One cause of interpatient variability that should be considered relates to proximal tubular secretion, which accounts for the majority of renal metformin clearance.16 In the pharmacokinetic literature, renal drug clearance is reported as a correlation with creatinine clearance rather than eGFR. Variable tubular secretion between patients with similar eGFRs could be an important source of interpatient variability. However, when we also analyzed our data using Cockroft-Gault estimates of creatinine clearance, no significant difference was found, and our results suggest that eGFR and creatinine clearance are equally accurate predictors of renal metformin clearance.
The strength of our trial is that, although relatively small, it still represents the largest prospective trial of pharmacokinetics in stage 4 CKD patients with repeated measures of metformin in steady-state conditions. The limitations of our study include its small numbers and relatively short duration of follow-up, self-reporting of compliance by patients as well as limited pharmacodynamic measures that were taken as part of an uncontrolled, open-label study.
We believe that the results of this study support the feasibility of extending the use of low-dose metformin to patients with stable stage 4 CKD and potentially beyond. Whereas metformin-associated lactic acidosis can be a fatal complication, it should be remembered that this is a very rare association, with rates of 4.3 episodes per 100,000 patient years.17 Perhaps even more important is the recognition that alternative treatments for diabetes in CKD have been shown to have even greater rates of serious side effects (such as significant hypoglycemic episodes) in multiple cross-sectional studies.18, 19, 20
However, although we believe that dose-adjusted use of metformin is a viable treatment option in more advanced stages of CKD, we also question whether the significant interpatient variability in metformin handling and the potential inaccuracies inherent in using eGFR as a surrogate for metformin tubular clearance make a case for more widespread use of metformin assays in routine clinical care. This assumes even greater importance in the setting of risk factors such as dehydration from acute kidney injury.
Although not in common use, the assay is relatively simple and affordable. Whereas it is not clear what therapeutic levels should be targeted, patients with normal renal function taking 2 g/d in 2 divided doses have been shown to run concentrations between 0.4 mg/l and 1.3 mg/l.21 Furthermore, it is recognized clinically and was demonstrated by Garber et al.22 in 1997 that the majority of the therapeutic benefit of metformin is provided by this 2-g dose with relatively little added benefit from higher doses. Moreover, even a 500-mg daily dose was found to be efficacious. Therefore, we would propose that metformin concentrations measured when on high-dose metformin in stage 3 CKD, although likely to be “supratherapeutic,” could still serve as personalized patient-specific safety data that will allow safe, but still therapeutic, dose reduction as CKD progresses. For example, a patient with an eGFR of 35 ml/min per 1.73 m2 taking 3 g of metformin who had a metformin trough concentration of ∼3 mg/l could have their dose of metformin adjusted down to “maintain” a level of 1 to 2 mg/l when their eGFR reaches levels <30. This would maintain therapeutic efficacy while ensuring that levels were maintained within empirically demonstrated, patient-specific safety thresholds.
In conclusion, we believe that our study is supportive of the liberalization of metformin use in stable moderate to severe CKD, as well as raising the question of the more widespread use of metformin assays to demonstrate tolerance of supratherapeutic but safe metformin concentrations and subsequent personalized metformin dosing.
Disclosure
All the authors declared no competing interests. The contents of this paper have not been published previously in whole or part, except in abstract form.
Acknowledgments
We are grateful to Middlemore clinical trials, Diabetes and Renal Services of Counties Manukau Health Auckland, New Zealand, for assistance with the conduct of this study; Dr. Michael Lam, nephrologist at Counties Manukau Health, who acted as the independent safety monitor; Dr Chris Florkowski and Christchurch Laboratories New Zealand for assisting with metformin measurements. Funding was provided by the diabetes fund at Middlemore clinical trials.
Footnotes
Supplementary Material. Description of a high-performance liquid chromatography (HPLC) assay that was used for the determination of metformin in plasma as has been described previously by Zhang et al.9
Supplementary material is linked to the online version of the paper at www.kireports.org.
Supplementary Material
Description of a high-performance liquid chromatography (HPLC) assay that was used for the determination of metformin in plasma as has been described previously by Zhang et al.9
References
- 1.Plantinga L.C., Crews D.C., Coresh J. Prevalence of chronic kidney disease in US adults with undiagnosed diabetes or prediabetes. Clin J Am Soc Nephrol. 2010;5:673–682. doi: 10.2215/CJN.07891109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American Diabetes Association 7. Approaches to glycemic treatment. Diabetes Care. 2016;39(Suppl 1):S52–S59. doi: 10.2337/dc16-S010. [DOI] [PubMed] [Google Scholar]
- 3.Inzucchi S.E., Bergenstal R.M., Buse J.B. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2015;38:140–149. doi: 10.2337/dc14-2441. [DOI] [PubMed] [Google Scholar]
- 4.Holman R.R. Type 2 diabetes mellitus in 2012: optimal management of T2DM remains elusive. Nat Rev Endocrinol. 2013;9:67–68. doi: 10.1038/nrendo.2012.243. [DOI] [PubMed] [Google Scholar]
- 5.FDA Drug Safety Communication: FDA revises warnings regarding use of the diabetes medicine metformin in certain patients with reduced kidney function. http://www.fda.gov/Drugs/DrugSafety/ucm493244.htm. Accessed December 5, 2016.
- 6.Metformin - Renal Impairment and Risk of Lactic Acidosis. 2015. http://www.medsafe.govt.nz/profs/PUArticles/December2015/Metformin.htm. Accessed December 5, 2016.
- 7.Inzucchi S.E., Lipska K.J., Mayo H. Metformin in patients with type 2 diabetes and kidney disease: a systematic review. JAMA. 2014;312:2668–2675. doi: 10.1001/jama.2014.15298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kajbaf F., De Broe M.E., Lalau J.D. Therapeutic concentrations of metformin: a systematic review. Clin Pharmacokinet. 2016;55:439–459. doi: 10.1007/s40262-015-0323-x. [DOI] [PubMed] [Google Scholar]
- 9.Zhang M., Moore G.A., Lever M. Rapid and simple high-performance liquid chromatographic assay for the determination of metformin in human plasma and breast milk. J Chromatogr B Analyt Technol Biomed Life Sci. 2002;766:175–179. doi: 10.1016/s0378-4347(01)00430-3. [DOI] [PubMed] [Google Scholar]
- 10.Julious S., Tan S.-B., Machin D. John Wiley & Sons Ltd; Somerset, NJ: 2010. An Introduction to Statistics in Early Phase Trials. [Google Scholar]
- 11.Sambol N.C., Chiang J., Lin E.T. Kidney function and age are both predictors of pharmacokinetics of metformin. J Clin Pharmacol. 1995;35:1094–1102. doi: 10.1002/j.1552-4604.1995.tb04033.x. [DOI] [PubMed] [Google Scholar]
- 12.Al-Hwiesh A.K., Abdul-Rahman I.S., El-Deen M.A. Metformin in peritoneal dialysis: a pilot experience. Perit Dial Int. 2014;34:368–375. doi: 10.3747/pdi.2013.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duong J.K., Roberts D.M., Furlong T.J. Metformin therapy in patients with chronic kidney disease. Diabetes Obes Metab. 2012;14:963–965. doi: 10.1111/j.1463-1326.2012.01617.x. [DOI] [PubMed] [Google Scholar]
- 14.Frid A., Sterner G.N., Londahl M. Novel assay of metformin levels in patients with type 2 diabetes and varying levels of renal function: clinical recommendations. Diabetes Care. 2010;33:1291–1293. doi: 10.2337/dc09-1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Graham G.G., Punt J., Arora M. Clinical pharmacokinetics of metformin. Clin Pharmacokinet. 2011;50:81–98. doi: 10.2165/11534750-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 16.Scheen A.J. Clinical pharmacokinetics of metformin. Clin Pharmacokinet. 1996;30:359–371. doi: 10.2165/00003088-199630050-00003. [DOI] [PubMed] [Google Scholar]
- 17.Salpeter S.R., Greyber E., Pasternak G.A., Salpeter E.E. Risk of fatal and nonfatal lactic acidosis with metformin use in type 2 diabetes mellitus. Cochrane Database Syst Rev. 2010;(4):CD002967. doi: 10.1002/14651858.CD002967. [DOI] [PubMed] [Google Scholar]
- 18.Arnouts P., Bolignano D., Nistor I. Glucose-lowering drugs in patients with chronic kidney disease: a narrative review on pharmacokinetic properties. Nephrol Dial Transplant. 2014;29:1284–1300. doi: 10.1093/ndt/gft462. [DOI] [PubMed] [Google Scholar]
- 19.Lalau J.D., Arnouts P., Sharif A., De Broe M.E. Metformin and other antidiabetic agents in renal failure patients. Kidney Int. 2015;87:308–322. doi: 10.1038/ki.2014.19. [DOI] [PubMed] [Google Scholar]
- 20.Bodmer M., Meier C., Krahenbuhl S. Metformin, sulfonylureas, or other antidiabetes drugs and the risk of lactic acidosis or hypoglycemia: a nested case-control analysis. Diabetes Care. 2008;31:2086–2091. doi: 10.2337/dc08-1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Timmins P., Donahue S., Meeker J., Marathe P. Steady-state pharmacokinetics of a novel extended-release metformin formulation. Clin Pharmacokinet. 2005;44:721–729. doi: 10.2165/00003088-200544070-00004. [DOI] [PubMed] [Google Scholar]
- 22.Garber A.J., Duncan T.G., Goodman A.M. Efficacy of metformin in type II diabetes: results of a double-blind, placebo-controlled, dose-response trial. Am J Med. 1997;103:491–497. doi: 10.1016/s0002-9343(97)00254-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of a high-performance liquid chromatography (HPLC) assay that was used for the determination of metformin in plasma as has been described previously by Zhang et al.9