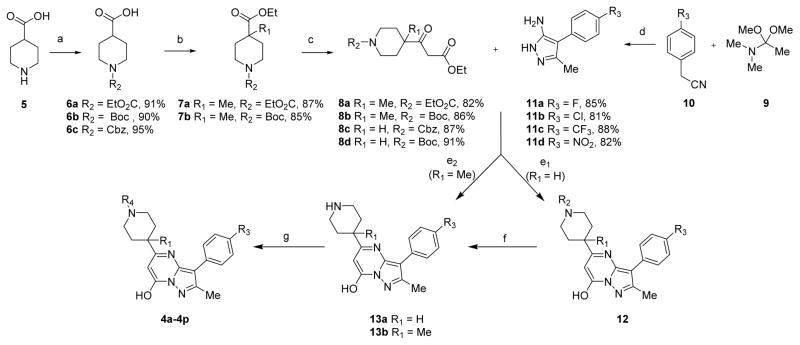
Scheme 1. Synthetic Route for Compound 4a and its Derivativesa.
aReagents and conditions: (a) R1O2CCl, NaOH, H2O, rt, 5 h, or (Boc)2O, K2CO3, THF/H2O, rt, 7 h; (b) (i) ClCO2Et, NEt3, rt, 12 h, (ii) n-BuLi, i-Pr2NH, THF, –78 °C, 1 h, MeI, THF, 5 h; (c) (i) 2 M NaOH, (ii) CDI, THF, 4 h, KO2CCH2CO2Et, MgCl2, DMAP, THF/CH3CN, overnight; (d) (i) DCM, microwave, 100 °C, (ii) N2H4·HCl, H2O, EtOH, 80 °C, 8 h; (e1) AcOH, reflux, 120 °C, overnight; (e2) EtOH, TFA, reflux, 85 °C, 48 h; (f) TFA, DCM, 0 °C, 2 h; (g) DMF, HOBt, HBTU, DIPEA, rt, overnight.