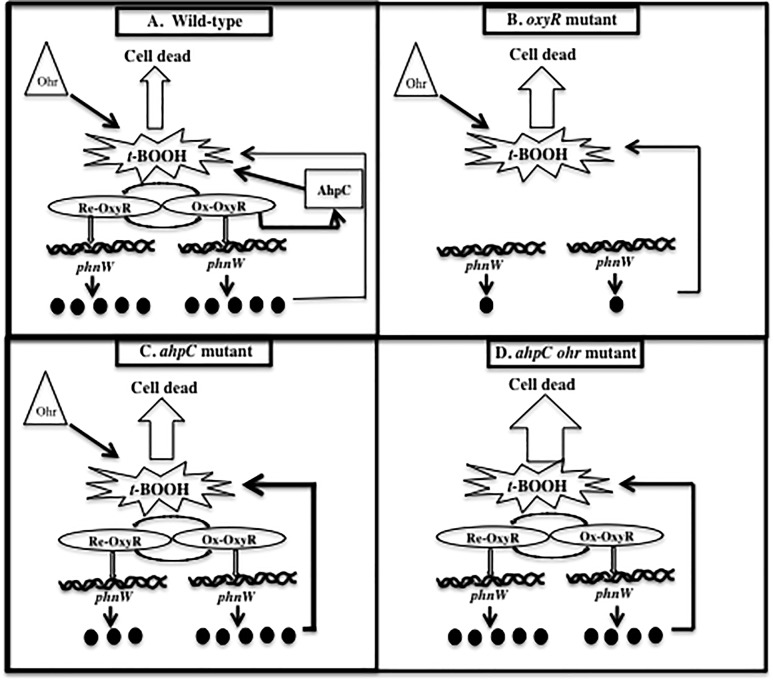
Fig 7. A model of phnW expression under difference conditions.
(A). In wild-type bacteria, phnW is continuously expressed in both untreated bacteria and during exposure to t-BOOH, while oxidized OxyR triggers activation of ahpC expression as previously shown [6]. Wild-type bacteria used both of these proteins and Ohr to assist in either the direct or indirect degradation of t-BOOH. This results in efficient t-BOOH degradation to a by-product that is not toxic to the bacteria, thereby preventing damage to proteins, DNA and lipid. (B). Expression of the phnW gene is significant lowered in oxyR mutant bacteria (80%) when compared to wild-type bacteria with no induction of ahpC expression when exposed to t-BOOH. This results in greater susceptibility to t-BOOH of this mutant relative to wild-type bacteria since this strain likely only uses Ohr to help detoxify t-BOOH. (C). Lower expression of phnW was also detected in a PAO1 ahpC mutant compared to wild type bacteria (30%). A lack of this major AHP in response to t-BOOH coupled with lower expression of phnW with only Ohr detoxifying power remaining results in less protection of this mutant from t-BOOH toxicity. (D). An ahpC ohr mutant showed a slightly reduced expression of phnW when bacteria were exposed to t-BOOH, but no difference were observed under control conditions when compared to wild-type expression. Thus, a lack of both of the major t-BOOH detoxification proteins and a lower expression of phnW results in this mutant being the most susceptible to t-BOOH. This may aid in bacterial protection from endogenous free radicals that are continuously generated under aerobic conditions and/or at the earliest time period, when exposed to t-BOOH while the responding gene is not yet expressed. When the bacteria are exposed to t-BOOH, oxidized OxyR governs over-expression of AhpCF to help in its detoxification and together with Ohr, an OxyR independent t-BOOH detoxification protein (Fig 7A). A significantly lower expression level of phnW gene (~80%) was revealed in the oxyR mutant under both reduced and oxidized conditions, indicative of OxyR-mediated regulation of the phnW gene (Figs 2 and 1). When the oxyR mutant that had significantly reduced expression of AhpC and PhnW was exposed to t-BOOH, this event triggered an increased susceptibility to this oxidant when compared to wild-type bacteria (Fig 7B). Interestingly, phnW expression levels were also ~30% lower when compared to wild-type levels but are complemented when exposed to t-BOOH in the ahpC but not in the ohr mutant (Fig 2). This could be yet another mechanism by which bacteria used to protect themselves from t-BOOH toxicity when they lack the major t-BOOH detoxifying protein, AhpC (Fig 7C). Both PA oxyR and PA ahpC mutants still have an Ohr (organic hydroperoxide resistance), one of the major proteins that can contribute to t-BOOH detoxification. A genome search for “peroxidase” and “hydroperoxide” revealed 6 and 5 hits, respectively. This indicates that there are likely multiple redundant mechanisms to dispose of hyperoxides such as t-BOOH. Therefore, we expected that phnW expression should be higher in the ohr ahpC double mutant to protect bacteria cell from t-BOOH. Surprisingly, expression was ~20% lower in this strain after exposure to t-BOOH. The lower expression of PhnW after exposure to t-BOOH in the ohr ahpC double mutant may also contribute to the sensitivity of this double mutant to t-BOOH (Fig 7D). AhpC is OxyR-dependenct but Ohr is not. Therefore, it appears that phnW expression levels depend on the present of OxyR in the cell and also the level of AhpC in ahpC or ahpC ohr mutants. Therefore, it is likely that OxyR directly regulated phnW expression level through binding on an upstream sequence of this gene in certain condition to help protect cell from t-BOOH toxicity (Figs 3 and 4). Frustratingly, though the mechanism of PhnW in responding to t-BOOH or regulated by OxyR is still unclear, this study clearly indicates that this protein has the ability to assist in the protection of PA cell from t-BOOH toxicity.