SUMMARY
Papillary thyroid carcinoma (PTC) is the most common malignant tumour of the thyroid. The effect of the concurrent presence of Hashimoto's thyroiditis (HT) and PTC is still under debate. The aim of this study is to investigate the influence of coexistent HT on prognostic outcomes and the association of coexistent HT with clinicopathological features. The demographic and clinicopathological data of 1,392 patients who underwent surgery in our hospital from 2007 to 2016 was collected and analysed. Among 1,392 PTC patients, the rate of HT was 25.6%. There were significant differences in the mean levels of thyroid stimulating hormone (3.27 vs. 2.41 μIU/L, p < 0.01), thyroperoxidase antibodies (110.31 vs. 131.2 U/ml, p < 0.01) and thyroglobulin antibodies (131.90 vs. 113.53 ng/ml, p < 0.01) between the two groups. PTC patients with HT had the following characteristics compared to patients without HT: smaller tumour size (p < 0.01), female predominance (p < 0.01) and higher rate of multifocality (p = 0.024). In addition, patients with HT had a significantly lower rate of lymph node metastasis (LNM) and advanced TNM stage than patients without HT (all p < 0.01). Multivariate analysis found that both age and multifocality were significantly associated with central LNM in HT patients (p < 0.01, p = 0.019, respectively). Extrathyroidal invasion and TSH level were also significant independent factors for lateral LNM in HT patients (p < 0.008, p = 0.04, respectively). HT is associated with a significantly higher risk of PTC. The coexistence of HT in PTC patients is associated with favourable clinical outcomes compared to PTC without HT. Total thyroidectomy and prophylactic central compartment lymphadenectomy should be a choice for PTC patients with HT.
KEY WORDS: Hashimoto's thyroiditis, Papillary thyroid cancer, Clinicopathologic characteristics
RIASSUNTO
Il carcinoma papillare (PTC) è il più comune tumore maligno della ghiandola tiroide. L'effetto della concomitante presenza della tiroidite di Hashimoto (HT) e del PTC è ancora oggetto di studio. Scopo di questo studio è analizzare la coesistenza di una concomitante HT circa l'outcome prognostico e eventuali associazioni clinico-patologiche. Abbiamo raccolto ed analizzato i dati demografici e clinicopatologici di 1392 pazienti che sono stati sottoposti a chirurgia nel nostro ospedale dal 2007 al 2016. Fra i 1392 pazienti con PTC, la percentuale di coesistente HT era del 25,6%. Vi erano differenze significative tra i due gruppi nei livelli medi di ormone tireostimolante (3.27 vs. 2.41μIU/L, p < 0.01), anticorpi anti tireoperossidasi (110.31 vs. 131.2U/ml, p < 0.01) e anticorpi anti tireoglobulina (131.90 vs. 113.53 ng/ ml, p < 0.01) I pazienti con PTC e HT avevano le seguenti caratteristiche se comparate con quelle dei pazienti senza HT: tumori di dimensioni più piccole (p < 0.01), predominanza del sesso femminile (p < 0.01) ed un piu' alto tasso di multifocalita'(p = 0.024). Inoltre, i pazienti con HT avevano un tasso significativamente basso di metastasi linfonodali (LNM) ed uno stadio di TNM più elevato rispetto ai pazienti senza HT (tutti p < 0.01). L'analisi multivariata ha evidenziato come età e multifocalità erano significativamente associate con metastasi nel compartimento centrale nei pazienti con HT (p < 0.01, p = 0.019, rispettivamente). L'invasione extratiroidea ed i livelli di TSH erano fattori significativamente indipendenti per le metastasi linfonodali laterocervicali nei pazienti con HT (p < 0.008, p = 0.04, rispettivamente). HT era associata ad un maggior rischio di sviluppare PTC. La coesistenza di HT in pazienti con PTC favoriva un miglior outcome clinico rispetto a quei pazienti con PTC ma senza HT. La tiroidectomia totale associata allo svuotamento del compartimento centrale deve essere la prima scelta chirurgica nei pazienti con PTC e HT.
Introduction
Thyroid cancer is the most common malignancy of the endocrine system, and its incidence rate is rapidly increasing by an average of 4.5% per year from 2007 to 2011 1. Approximately 70% to 80% of thyroid cancers are papillary thyroid carcinomas (PTCs), which exhibited a relatively benign clinical course 2 3. Hashimoto's thyroiditis (HT), also called chronic lymphocytic or autoimmune thyroiditis, is the most common inflammatory thyroid disease. The incidence worldwide is estimated to be 0.3-1.5 cases per 1000 individuals 4. This autoimmune disease is the most common cause of primary hypothyroidism and non-endemic goiter, and the incidence is estimated to be 10-15 times higher in women. In China, the rate is higher, with approximately 0.4%-1.5% of the population affected, which accounts for 20%-25% of all thyroid disease 5-7. Since its first description by Dailey et al. in 1955 8, many aetiological and epidemiological studies have investigated the relationship between PTC and HT. Some authors have demonstrated that PTC with HT is associated with pathologic factors that indicate decreased tumour aggressiveness, such as small tumour size and low stage. It has also been associated with lower rates of recurrence, better locoregional control and longer overall survival 8-11. Other authors have shown no relationship between the presence of HT and tumour aggressiveness 12-15. Nowadays, the correlation between the two diseases with regards to pathogenesis and prognostic outcomes is still unclear.
Given the relatively high incidence of both diseases and the ongoing debate, we undertook a retrospective study to investigate the potential relationship between PTC and HT, and the effect of coexistent HT on the presentation, management and clinical outcomes of PTC patients.
Patients and methods
Between January 2007 and April 2016, there were 7,354 patients who underwent thyroid surgery in Liaoning Cancer Hospital & Institute. In the pathological review of these patients, 5,844 had benign lesions and 1,510 had malignant tumours. There were 1,392 PTCs, 58 follicular thyroid cancers, 23 medullary thyroid cancers, 15 lymphomas, 10 squamous cell carcinomas and 12 undifferentiated carcinomas. Among all patients, 1,682 were diagnosed as having pathological changes consistent with HT, while 5,672 were identified without HT. Pathologically-demonstrated HT was defined as the presence of diffuse lymphoplasmacytic infiltration, germinal centres and enlarged epithelial cells with large nuclei and eosinophilic cytoplasm. Only peri-tumoural lymphocytic infiltration was not regarded as HT.
Thyroid lobectomy with isthmusectomy was performed in 520 patients, whereas total thyroidectomy was performed in 872 cases. During lymph node resection, the central compartment was delimited superiorly by the hyoid bone, inferiorly by the substernal notch, laterally by the median portion of the carotid sheath and dorsally by the prevertebral fascia. Central neck dissection without lateral compartment neck dissection was performed in 785 patients. Comprehensive neck dissections such as radical neck dissection and modified neck dissection were performed in 495 patients, and 143 of these underwent bilateral neck dissection.
The following variables were considered: age, gender, thyroid function tests, fine needle aspiration biopsy (FNAB), tumour size, multifocality, extrathyroidal invasion, extension of surgery, lymph node metastasis (LNM), TNM stage, recurrence and distant metastasis. Patients were staged according to the seventh edition of the UICC/AJCC TNM staging system 16. This study was approved by ethical committees of our hospital, and informed consent was obtained from each patient. In addition, the AMES clinical staging system and the MACIS scoring system were used to evaluate the prognostic outcome. The AMES staging system divides patients into two groups: low risk (i) females < 51 years and males < 41 years without distant metastasis, and (ii) elderly patients with tumours < 5 cm with no extrathyroidal extension of the papillary carcinoma and high risk (i) patients with distant metastasis and (ii) females ≥ 51 years and males ≥ 41 years with tumours ≥ 5 cm or extrathyroidal extension if it is papillary carcinoma 17. The MACIS staging system has established that high score is strongly correlated with poor prognosis. It is obtained by adding 3.1 if the patient is ≤ 39 years or 0.08 × age if the patient is > 40 years, +0.3 × tumour size in cm, +1 if the tumour is not completely resectable, +1 if it is locally invasive and +3 in the presence of distant metastasis. Patients are divided into four groups: group 1, < 6; group 2, 6-6.99; group 3, 7-7.99; and group 4, ≥ 8 18.
Preoperative diagnostic evaluation
Diagnosis and preoperative evaluation of each patient were performed according to a strategy that was not changed during the study period. All patients underwent careful history and thorough physical examination in our department. All patients who qualified for surgical treatment were subjected to thyroid ultrasonography, determinations of free thyroid hormones (T3, T4) and thyroid stimulating hormone (TSH), as well as thyroperoxidase antibodies (TPOAb) and thyroglobulin antibodies (TgAb). FNAB and ultrasonography-guided FNAB were also used. A suspicious malignant nodule was diagnosed in the presence of at least one of the following ultrasound images: micro-calcification, infiltrative margin, increased nodular vascularity, taller that wide on transverse view and hypo-echoic. Metastases to the lung and lymph nodes were evaluated by preoperative imaging studies, such as CT.
Follow-up
Patient progress was followed by physical examination, ultrasonography and CT to identify recurrence. Furthermore, we also used FNAB on suspected masses or lymph nodes, and cytopathologic diagnosis was obtained. All patients were closely followed after surgery until August 2016. The median follow-up duration of patients was 38.4 months (range, 3.1-125.3).
Statistical analyses
All statistical analyses were performed using the SPSS 16.0 statistical package (SPSS, Inc., Chicago, IL, USA). Cancer-specific survival was analysed using Kaplan- Meier survival curves, and comparisons were made using the log-rank test. In univariate analysis, two-tailed χ2 were used for statistical comparisons. In multivariate analysis, logistic regression analysis applied to identify the significant clinicopathologic factors correlated with LNM. For all analyses, only p values < 0.05 were considered significant.
Results
In total, 25.6% of patients with PTC (357/1,392) had coexisting HT. The proportion of female patients in the HT group was higher than that in the non-HT group (91.6% vs. 65.2%, p < 0.01). More PTC was found in patients with HT than in those without HT (21.2% vs. 18.2%, p = 0.007) (Table I). Of the 1,392 PTC patients, there were 278 males (20.0%) and 1,114 females (80.8%; ratio 1:4) with a mean age 45.04 ± 12.47 years (median 45 years; range 10-82 years). The mean tumour size was 1.85 ± 1.11 cm. The sensitivity of FNAB was 49.5%. In our study, 373 patients had multifocality, and extrathyroidal invasion was identified in 34.8% of the patients. Central lymph node involvement was identified in 295 patients, lateral lymph node involvement in 198 patients and central and lateral LNM in 182 patients. During lymphadenectomy, one to 34 lymph nodes were removed. The number of involved lymph nodes varied between 0 and 22. There were 896 patients with stage I disease (64.3%), 58 with stage II (4.2%), 242 with stage III (17.4%) and 196 with stage IV (14.1%).
Table I.
Demographic information of 7,354 patients.
| Variables | HT (n = 1,682) | Non-HT (n = 5,672) | P value |
|---|---|---|---|
| Age (years) | 49.34 ± 11.33 | 49.98 ± 11.85 | 0.34 |
| Gender | < 0.01 | ||
| Male | 141 (8.4%) | 1,975 (34.8%) | |
| Female | 1,541 (91.6%) | 3,697 (65.2%) | |
| With PTC | 357 (21.2%) | 1,035 (18.2%) | 0.007 |
Patients with PTC and HT appeared to be slightly younger than those without HT (mean age 44.14 ± 11.95 vs. 45.34 ± 12.63); this difference was not statistically significant (p = 0.197). A greater female preponderance was noted in the patients with HT compared with those without HT (p < 0.01). Compared with non-HT group, the patients with HT group had higher levels of preoperative TSH, TgAb and TPOAb (all p < 0.01). Mean tumour size in patients with HT was smaller than in those without HT (p < 0.01). Additionally, the rate of multifocality was significantly different between the two groups (31.4% vs. 25.2%, p = 0.024). There was no difference in extrathyroidal extension between the two groups (p = 0.085). In HT patients, central LNM had a lower frequency compared with non-HT patients (19.6% vs. 21.7%, p < 0.01). Patients with HT had a significantly lower frequency of advance-stage disease (p < 0.01). However, no significant differences were found in terms of recurrence (p = 0.787) and distant metastasis (p = 0.06) between the two groups. The clinicopathological characteristics of 1,392 patients are summarised in Table II.
Table II.
Clinicopathologic characteristics of 1,392 patients with PTC stratified by the presence of HT.
| Variables | PTC with HT (n = 357) | PTC without HT (n = 1,035) | P value |
|---|---|---|---|
| Age (years) | 44.14 ± 11.95 | 45.34 ± 12.63 | 0.197 |
| Gender (male:female) | 1:9.5 | 1:3.2 | < 0.01 |
| TSH (μIU/L) | 3.27 ± 5.46 | 2.41 ± 3.34 | < 0.01 |
| TgAb (ng/ml) | 131.90 ± 348.92 | 113.53 ± 206.21 | < 0.01 |
| TPOAb (U/ml) | 110.31 ± 171.83 | 131.2 ± 97.54 | < 0.01 |
| Tumour size (cm) | 1.58 ± 0.97 | 1.94 ± 1.14 | < 0.01 |
| ≤ 1 | 151 (42.3%) | 280 (27.1%) | |
| > 1 | 206 (57.7%) | 755 (72.9%) | |
| Multifocality | 112 (31.4%) | 261 (25.2%) | 0.024 |
| Extrathyroidal invasion | 111 (31.1%) | 374 (36.1%) | 0.085 |
| Lymph node metastasis | < 0.01 | ||
| Central only | 70 (19.6%) | 225 (21.7%) | |
| Lateral only | 34 (9.5%) | 164 (15.8%) | |
| Central + lateral | 45 (12.6%) | 137 (13.2%) | |
| TNM staging | < 0.01 | ||
| StageI | 252 (70.6%) | 644 (62.2%) | |
| Stage II | 7 (2.0%) | 51 (4.9%) | |
| Stage III | 67 (18.8%) | 175 (17.0%) | |
| StageIV | 31 (8.6%) | 165 (15.9%) | |
| Recurrence | 13(3.6%) | 41 (4.0%) | 0.787 |
| Distant metastasis | 2 (0.6%) | 21 (2.0%) | 0.06 |
A multivariate logistic regression analysis that included age, gender, tumour size, multifocality, extrathyroidal invasion and TSH level was performed to assess whether these clinicopathological factors were associated with LNM in PTC patients with HT. We found that age and multifocality were significantly associated with central LNM in HT patients (p < 0.01, p = 0.019) (Table III). Next, we investigated the risk factors associated with lateral LNM in PTC patients with HT. Extrathyroidal invasion and TSH level were significant independent factors for lateral LNM in HT patients, with odds ratio of 0.353 (95% CI, 0.164-0.757, p < 0.008), 2.223 (95% CI, 1.038- 4.757, p = 0.04) (Table IV).
Table III.
Univariate and multivariate analysis for central LNM with statistically significant variables.
| Variables | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| N | P value | OR | 95% CI | P value | |
| Age (year) | < 0.01 | 0.334 | 0.193-0.576 | < 0.01 | |
| < 45 | 123 (44.2%) | ||||
| ≥ 45 | 155 (55.8%) | ||||
| Gender | 0.097 | ||||
| Male | 23 (8.3%) | ||||
| Female | 255 (91.7%) | ||||
| Tumour size | 0.154 | ||||
| ≤ 1 | 128 (46.0%) | ||||
| > 1 | 150 (54.0%) | ||||
| Multifocality | 79 (28.4%) | 0.021 | 2.002 | 1.118-3.583 | 0.019 |
| Extrathyroidal invasion | 81 (29.1%) | 0.063 | |||
| TSH | 0.293 | ||||
| < 2.5 | 168 (60.4%) | ||||
| ≥ 2.5 | 110 (39.6%) | ||||
Table IV.
Univariate and multivariate analysis for lateral LNM with the statistically significant variables.
| Variables | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| N | P value | OR | 95% CI | P value | |
| Age (year) | 0.053 | ||||
| < 45 | 142 (45.6%) | ||||
| ≥ 45 | 170 (54.4%) | ||||
| Gender | 0.215 | ||||
| Male | 28 (9.0%) | ||||
| Female | 284 (91%) | ||||
| Tumour size | 0.130 | ||||
| ≤ 1 | 139 (44.6%) | ||||
| > 1 | 173 (55.4%) | ||||
| Multifocality | 89 (28.5%) | 0.904 | |||
| Extrathyroidal invasion | 97 (31.1%) | 0.033 | 0.353 | 0.164-0.757 | 0.008 |
| TSH | 0.032 | 2.223 | 1.038-4.757 | 0.04 | |
| < 2.5 | 182 (58.3%) | ||||
| ≥ 2.5 | 130 (41.7%) | ||||
Two well-established prognosis classification systems of PTC patients were used. Using the AMES staging system, the rate of high risk group of PTC patients without HT was slightly higher than that of patients with HT (18.6% vs. 14.8%). However, no significant difference was found between the two groups (p = 0.113). According to the MACIS scoring system, the trend was more evident between the two groups (13.8% vs. 8.7%, p = 0.012). The PTC patients without HT had a higher mean score than those with HT (4.80 vs. 4.52, p < 0.01) (Table V).
Table V.
AMES stage and MACIS score of 1,392 patients with PTC stratified by the presence of HT.
| PTC with HT (n = 357) |
PTC alone (n = 1,035) |
P value | |
|---|---|---|---|
| AMES stage | 0.113 | ||
| Low risk | 304 (85.2%) | 843 (81.4%) | |
| High risk | 53 (14.8%) | 192 (18.6%) | |
| MACIS score | |||
| Mean | 4.52 ± 0.95 | 4.80±1.16 | < 0.01 |
| ≤ 6 | 326 (91.3%) | 892 (86.2%) | 0.012 |
| > 6 | 31 (8.7%) | 143 (13.8%) |
During the follow-up period, 13 cases (3.6%) experienced recurrence in the HT group: 6 had thyroid recurrence and 7 had lymph node recurrence. In patients without HT, a total of 41 patients (4.0%) developed recurrence: 16 had thyroid recurrence and 25 had lymph node recurrence. All these patients underwent re-operation, and they all remain alive with no symptoms of further recurrence after second surgery. There were 2 patients who had lung metastasis in the HT group. In patients without HT, 20 had lung metastasis, while one had bone metastasis. Overall, one patient in the HT group and 10 patients in the non-HT group died, but only 3 of these deaths were due to PTC or related complications. The patients with HT tended to have better prognosis compared with that of patients without HT, and disease-free survival (DFS) in patients with HT was significantly higher than that without HT (p = 0.009, Fig. 1). However, the difference was not statistically significant when considering overall survival (OS) between the two groups (p = 0.706, Fig. 2).
Fig. 1.
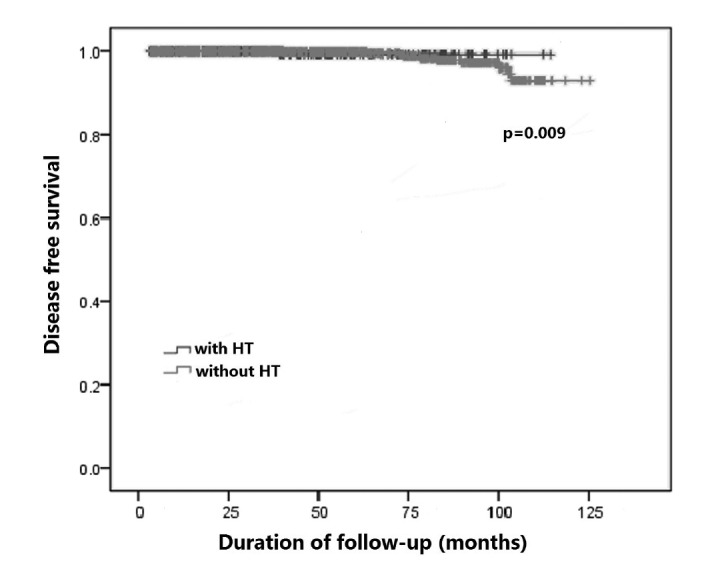
Comparison of disease-free survival between groups.
Fig. 2.
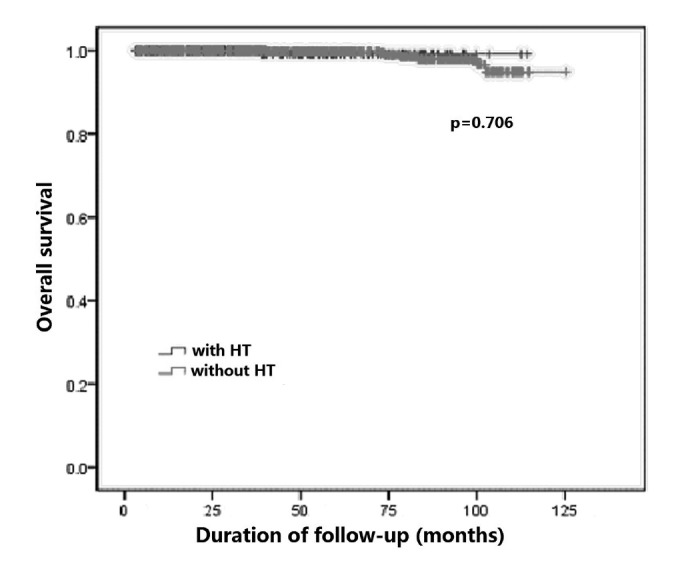
Comparison of overall survival between groups.
Discussion
The thyroid gland is affected by autoimmune attacks more than any other organ, with HT being the most common thyroidal autoimmune disease. HT is regarded as a destructive tissue-specific autoimmune disease with detectable TPOAb and TgAb. It is characterised by widespread lymphocyte infiltration, fibrosis and parenchymal atrophy of thyroid tissue. The disease usually leads to hypothyroidism, which is characterised by a deficit of T3 and T4 and elevated TSH levels. A visible increase in the incidence of co-existent PTC and HT has been found during the past 20 years, and the association between the two diseases has been a topic of discussion. According to abundant data from the literature and a meta-analysis performed by Singh et al., PTC coexisted with HT 2.8 more frequently and its prevalence in various studies ranged from 0.5% to 38.0% 7 19-21. PTC was also found to occur almost twice as often as other types of thyroid cancer 22. The observed variability in the rates could be explained by ethnic, geographic, patient characteristics and environmental factors. Moreover, the variability might also be attributed to differences in the pathologic definitions and histopathologic interpretation of HT used 20 23. In this study, we found a similar rate (25.6%) as reported by others. Long-term HT frequently leads to hypothyroidism, which elevates TSH levels. Thus, when considering whether HT is a risk factor for PTC, it is necessary to investigate the levels of TSH. TSH is known to have a trophic effect on follicular-cell thyroid cancer and those of follicular-cell origin 24. Elevated TSH might potentially increase the risk of thyroid tumour because of TSH-induced proliferation of thyroid cells 25. Some authors have proposed that the development of thyroid autonomy, reducing TSH levels, may slow cancer progression 26. In our study, we found that the rate of PTC in patients with HT (21.2%) was much higher than in those without HT (18.2%), and TSH levels in HT patients were significantly higher compared to those without HT. Theses results indicate that HT might be a potential risk factor for PTC patients. We hypothesised that long-term HT causes elevated TSH, which probably is the main factor responsible for PTC. This hypothesis might also explain why some prospective studies had negative results in terms of the relationship between PTC and HT 10 19 22. Thus, in clinical practice, patients with thyroid nodules and who are suspected of having HT need to be carefully monitored since the possibility of malignancy is increased.
There are conflicting results with regards to the biologic behaviour of PTC in the presence of HT. Some studies have reported that the presence of HT in PTC patients has been related to lower T stage, less extrathyroidal invasion and less nodal metastasis compared to patients without HT in previous studies 9-11. Other studies have shown HT does not influence any prognostic factors such as size, extrathyroidal extension, or multifocality, thus showing no relationship between HT and PTC aggressiveness 7 15 20. Our results revealed that PTC patients with HT were significantly more frequent in the population of females in a lower age range, presenting with small lesion and multifocal disease, but also with statistically less extrathyroidal extension. Moreover, PTC patients with HT had earlystage disease and less LNM at the time of surgery. Although no significant differences were found in terms of recurrence and distant metastasis, the rates of both were lower in HT patients.
In recent years, many researchers have tried to elucidate the relationship between PTC and HT from pathophysiological standpoint. The proto-oncogene RET, which is located on chromosome 10q11.2 and encodes a transmembrane receptor-tyrosine kinase, might play an important role between PTC and HT development by RET/PTC rearrangement 27. This rearrangement has been described in the large majority of tissue with HT and without detectable PTC, which might exhibit progression to cancer from chronic thyroiditis 27 28. Some authors proposed that the mitogen-activated protein kinase signalling pathway, which is activated by the RET/PTC rearrangement, is crucial in the relationship between both diseases. Mutations in the BRAF gene are also common in thyroid tumours 29. Franco et al. reported that mice with thyroidspecific knockin of oncogenic BRAF present invasive thyroid cancer and have high TSH levels. However, when they were crossed with TSH-receptor knockout mice, the BRAF mutated gene was not able to induce cancer 30.
Currently, few studies have investigated the effect of HT and the BRAFV600E mutation on PTC patients. Additionally, Larson et al. found the PI3K/Akt signal pathway was highly activated in HT and thyroid cancer tissue, and they proposed that this is a molecular mechanism leading to carcinogenesis in HT 31. Further investigations are needed to explain the relationship between HT and these genes in PTC patients.
Although recent studies have investigated the impact of HT on PTC tumour behaviour, only several studies have reported its association with LNM 32-34. Jeong et al. found no difference in central LNM between PTC patients with and without HT, but they did not investigate additional factors associated with LNM 32. Kim et al. suggested that HT associated with PTC may protect against central LNM, and there was no significant association between the coexistence of HT and central and lateral LNM 33. We found that PTC patients with HT were associated with a low rate of central and lateral LNM (19.6% vs. 9.5%). It was suggested that the autoimmune response to thyroid specific antigens in patients HT might be involved in destruction of cancer cells expressing thyroid specific antigen in PTC, thus preventing recurrence and LNM. Additionally, we noticed that age and multifocality was significantly associated with central LNM in HT patients in multivariable analysis, and extrathyroidal invasion and TSH level were independent factors for lateral LNM. These results indicated a potential protective role of autoimmune thyroiditis in lymphatic tumour spread. However, the explanation for the difference in the rate of central and lateral LNM on the basis of whether HT is present is unknown.
Many studies have demonstrated that PTC patients with HT have better prognosis. Some authors revealed a positive correlation between HT in PTC patients and DFS and OS; hence, they concluded that these patients had a more favourable prognosis 7. Kashima et al. reported a mortality and 10-year DFS of 0.7% and 95% in HT patients, compared to 5% and 85% non-HT patients, respectively 11. In our cohort, PTC patients with HT tended to have a more indolent clinical course compared to those with PTC alone, including a lower rates of OS and DFS; however, the differences in overall survival did not reach statistical significance. Because prognosis of PTC is remarkably excellent, it is sometimes difficult to analyse survival differences between subgroups of PTC. At present, the AMES stage and MACIS scoring system are most commonly adopted for predicting survival and formulating selective treatment strategies for thyroid cancer patients. Thus, we also analysed prognostic outcomes separately for the low and high risk groups by AMES stage and MACIS scoring system. The results showed that the proportion of low risk patients with PTC and HT was higher, and these patients also had lower MACIS scores. Although some p-values did not reach statistical significance, our findings could possibly change with additional patients and a longer follow-up period.
HT by itself is not an indication for surgery, but concurrent malignancy or the presence of goiter should be treated by surgery as the preferred option. As for the extent of surgery in these patients, some authors are inclined to have total thyroidectomy to eliminate the possibility of a potential cancer 34-37. Total thyroidectomy not only allowed for treating a disease already diagnosed based on FNAB, but also contributed to decreasing the rate of reoperation due to postoperative diagnosis of thyroid cancer. The various therapeutic strategies employed in HT patients have led the present surgeons to present their own opinion. PTC is the most common thyroid cancer with the predilection for lymphatic spread, and central lymph nodes are usually in the target area in differentiated thyroid cancer with LNM 38. Thus, it is our belief that in view of the relatively high rate of PTC in HT, the strategy of surgical treatment of HT in these patients might include total thyroidectomy and prophylactic central compartment lymphadenectomy. Even if a second surgery is needed because of neck recurrence, a neck lymph node dissection will be sufficient without increasing the operational difficulty and risks of hoarseness and hypocalcaemia.
We acknowledge that there are several limitations in our study. First, it was retrospective and as such was limited by the content, accuracy and availability of the clinical records utilised. Further longitudinal prospective studies are required to assess the potential relationship between the two diseases, if any, and the pathogenetic mechanisms involved. Second, in some studies, HT and PTC may share a possible risk factor, namely excessive intake of iodine, and it has also been proposed that changes in iodine intake might be responsible for the increase of PTC with HT 39. However, we were not able to perform more detailed assessment for lifestyle, such as dietary iodine intake, and this potential confounding parameter for the relationship between PTC and HT were not investigated fully. In a recent study, the BRAFV600E mutation was present in 72.1% of HT patients with PTC and the rate was significantly lower compared to 81.1% found in patients without HT 40. Since it was not investigated in our study, it can be considered as another limitation. In addition, it is very difficult for us to assess the mean time of HT in patients with PTC. It is necessary that the studies on increased TSH causing PTC in HT patients should be investigated in the near future, which may provide more information for better comprehension of the relationship between PTC and HT.
In conclusion, we found a relatively common occurrence of HT in patients with PTC. Compared to patients with PTC alone, patients with HT were younger, predominantly female, had a smaller tumour size, multifocal and low stage disease at the time of surgery. Simultaneously, our results showed that HT may influence LNM in PTC patients. HT was associated with a reduced of central and lateral LNM, which indicated a potential protective effect. More studies on the immunoregulatory mechanism and molecular mechanisms, such as high iodine intake, mutations in proto-oncogenes, balance of cell proliferation and activation of kinase activity, are still needed to support or refute this association.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Jiwang L, Zhendong L, Shuchun L, et al. Clinicopathologic characteristics of familial versus sporadic papillary thyroid carcinoma. Acta Otorhinolaryngol Ital. 2015;35:234–242. [PMC free article] [PubMed] [Google Scholar]
- 3.Cho BY, Choi HS, Park YJ, et al. Changes in the clinicopathological characteristics and outcomes of thyroid cancer in Korea over the past four decades. Thyroid. 2013;23:797–804. doi: 10.1089/thy.2012.0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vanderpump MP, Tunbridge WM, French JM, et al. The incidence of thyroid disorders in the community: a twenty-year follow-up of the Whickham Survey. Clin Endocrinol (Oxf) 1995;43:55–68. doi: 10.1111/j.1365-2265.1995.tb01894.x. [DOI] [PubMed] [Google Scholar]
- 5.Jankovic B, Le KT, Hershman JM. Clinical review: Hashimoto’s thyroiditis and papillary thyroid carcinoma: is there a correlation? J Clin Endocrinol Metab. 2013;98:474–482. doi: 10.1210/jc.2012-2978. [DOI] [PubMed] [Google Scholar]
- 6.Teng W, Shan Z, Teng X, et al. Effect of iodine intake on thyroid disease in China. Effect of iodine intake on thyroid diseases in China. N Engl J Med. 2006;354:2783–2793. doi: 10.1056/NEJMoa054022. [DOI] [PubMed] [Google Scholar]
- 7.Singh B, Shaha AR, Trivedi H, et al. Coexistent Hashimoto's thyroiditis with papillary thyroid carcinoma: impact on presentation, management, and outcome. Surgery. 1999;126:1070–1106. doi: 10.1067/msy.2099.101431. [DOI] [PubMed] [Google Scholar]
- 8.Dailey ME, Lindsay S, Skahen R. Relation of thyroid neoplasms to Hashimoto disease of the thyroid gland. AMA Arch Surg. 1955;70:291–297. doi: 10.1001/archsurg.1955.01270080137023. [DOI] [PubMed] [Google Scholar]
- 9.Kim KW, Park YJ, Kim EH, et al. Elevated risk of papillary thyroid cancer in Korean patients with Hashimoto's thyroiditis. Head Neck. 2011;33:691–695. doi: 10.1002/hed.21518. [DOI] [PubMed] [Google Scholar]
- 10.Repplinger D, Bargren A, Zhang YW, et al. Is Hashimoto's thyroiditis a risk factor for papillary thyroid cancer? J Surg Res. 2008;150:49–52. doi: 10.1016/j.jss.2007.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kashima K, Yokoyama S, Noguchi S, et al. Chronic thyroiditis as a favorable prognostic factor in papillary thyroid carcinoma. Thyroid. 1998;8:197–202. doi: 10.1089/thy.1998.8.197. [DOI] [PubMed] [Google Scholar]
- 12.Anil C, Goksel S, Gursoy A. Hashimoto's thyroiditis is not associated with increased risk of thyroid cancer in patients with thyroid nodules: a single-center prospective study. Thyroid. 2010;20:601–606. doi: 10.1089/thy.2009.0450. [DOI] [PubMed] [Google Scholar]
- 13.Haymart MR, Repplinger DJ, Leverson GE, et al. Higher serum thyroid stimulating hormone level in thyroid nodule patients is associated with greater risks of differentiated thyroid cancer and advanced tumor stage. J Clin Endocrinol Metab. 2008;93:809–814. doi: 10.1210/jc.2007-2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holm LE, Blomgren H, Löwhagen T. Cancer risks in patients with chronic lymphocytic thyroiditis. N Engl J Med. 1985;312:601–604. doi: 10.1056/NEJM198503073121001. [DOI] [PubMed] [Google Scholar]
- 15.Del Rio P, Cataldo S, Sommaruga L, et al. The association between papillary carcinoma and chronic lymphocytic thyroiditis: does it modify the prognosis of cancer? Minerva Endocrinol. 2008;33:1–5. [PubMed] [Google Scholar]
- 16.Edge SB, Byrd DR, Compton CC, et al. AJCC cancer staging manual. 7th ed. New York: Springer; 2010. [Google Scholar]
- 17.Sanders LE, Cady B. Differentiated thyroid cancer: reexamination of risk groups and outcome of treatment. Arch Surg. 1998;133:419–425. doi: 10.1001/archsurg.133.4.419. [DOI] [PubMed] [Google Scholar]
- 18.Hay ID, Bergstralh EJ, Goellner JR, et al. Predicting outcome in papillary thyroid carcinoma: development of a reliable prognostic scoring system in a cohort of 1779 patients surgically treated at one institution during 1940 through 1989. Surgery. 1993;114:1050–1057. [PubMed] [Google Scholar]
- 19.Kim EY, Kim WG, Kim WB, et al. Coexistence of chronic lymphocytic thyroiditis is associated with lower recurrence rates in patients with papillary thyroid carcinoma. Clin Endocrinol (Oxf) 2009;71:581–586. doi: 10.1111/j.1365-2265.2009.03537.x. [DOI] [PubMed] [Google Scholar]
- 20.Kebebew E, Treseler PA, Ituarte PH, et al. Coexisting chronic lymphocytic thyroiditis and papillary thyroid cancer revisited. World J Surg. 2001;25:632–637. doi: 10.1007/s002680020165. [DOI] [PubMed] [Google Scholar]
- 21.Okayasu I, Fujiwara M, Hara Y, et al. Association of chronic lymphocytic thyroiditis and thyroid papillary carcinoma. A study of surgical cases among Japanese, and white and African Americans. Cancer. 1995;76:2312–2318. doi: 10.1002/1097-0142(19951201)76:11<2312::aid-cncr2820761120>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 22.Cipolla C, Sandonato L, Graceffa G, et al. Hashimoto thyroiditis coexistent with papillary thyroid carcinoma. Am Surg. 2005;71:874–878. [PubMed] [Google Scholar]
- 23.Loh KC, Greenspan FS, Dong F, et al. Influence of lymphocytic thyroiditis on the prognostic outcome of patients with papillary thyroid carcinoma. J Clin Endocrinol Metab. 1999;84:458–463. doi: 10.1210/jcem.84.2.5443. [DOI] [PubMed] [Google Scholar]
- 24.Keshin M, Savas-Erdeve S, Aycan Z. Co-existence of thyroid nodule and thyroid cancer in children and adolescents with Hashimoto thyroiditis: a single-center study. Horm Res Paediatr. 2016;85:181–187. doi: 10.1159/000443143. [DOI] [PubMed] [Google Scholar]
- 25.Fiore E, Rago T, Latrofa F, et al. Hashimoto's thyroiditis is associated with papillary thyroid carcinoma: role of TSH and of treatment with L-thyroxine. Endocr Relat Cancer. 2011;18:429–437. doi: 10.1530/ERC-11-0028. [DOI] [PubMed] [Google Scholar]
- 26.Fiore E, Rago T, Provenzale MA, et al. Lower levels of TSH are associated with a lower risk of papillary thyroid cancer in patients with thyroid nodular disease: thyroid autonomy may play a protective role. Endocr Relat Cancer. 2009;16:1251–1260. doi: 10.1677/ERC-09-0036. [DOI] [PubMed] [Google Scholar]
- 27.Wirtschafter A, Schmidt R, Rosen D, et al. Expression of the RET/PTC fusion gene as a marker for papillary carcinoma in Hashimoto's thyroiditis. Laryngoscope. 1997;105:95–100. doi: 10.1097/00005537-199701000-00019. [DOI] [PubMed] [Google Scholar]
- 28.Arif S, Blanes A, Diaz-Cano SJ. Hashimoto's thyroiditis shares features with early papillary thyroid carcinoma. Histopathology. 2002;41:357–362. doi: 10.1046/j.1365-2559.2002.01467.x. [DOI] [PubMed] [Google Scholar]
- 29.Xing M. BRAF mutation in papillary thyroid cancer: pathogenic role, molecular bases, and clinical implications. Endocr Rev. 2007;28:742–762. doi: 10.1210/er.2007-0007. [DOI] [PubMed] [Google Scholar]
- 30.Franco AT, Malaguarnera R, Refetoff S, et al. Thyrotrophin receptor signaling dependence of Braf-induced thyroid tumor initiation in mice. Proc Natl Acad Sci U S A. 2011;108:1615–1620. doi: 10.1073/pnas.1015557108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Larson SD, Jackson LN, Riall TS, et al. Increased incidence of well-differentiated thyroid cancer associated with Hashimoto thyroiditis and the role of the PI3k/Akt pathway. J Am Coll Surg. 2007;204:764–773. doi: 10.1016/j.jamcollsurg.2006.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jeong JS, Kim HK, Lee CR, et al. Coexistence of chronic lymphocytic thyroiditis with papillary thyroid carcinoma: clinical manifestation and prognostic outcome. J Korean Med Sci. 2012;27:883–889. doi: 10.3346/jkms.2012.27.8.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim SS, Lee BJ, Lee JC, et al. Coexistence of Hashimoto's thyroiditis with papillary thyroid carcinoma: the influence of lymph node metastasis. Head Neck. 2011;33:1272–1277. doi: 10.1002/hed.21594. [DOI] [PubMed] [Google Scholar]
- 34.Konturek A, Barczyński M, Wierzchowski W, et al. Coexistence of papillary thyroid cancer with Hashimoto thyroiditis. Langenbecks Arch Surg. 2013;398:389–394. doi: 10.1007/s00423-012-1021-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Serpell JW, Phan D. Safety of total thyroidectomy. ANZ J Surg. 2007;77:15–19. doi: 10.1111/j.1445-2197.2006.03897.x. [DOI] [PubMed] [Google Scholar]
- 36.Anand A, Singh KR, Kushwaha JK, et al. Papillary thyroid cancer and Hashimoto's thyroiditis: an association less understood. Indian J Surg Oncol. 2014;3:199–204. doi: 10.1007/s13193-014-0325-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kurukahvecioglu O, Taneri F, Yüksel O, et al. Total thyroidectomy for the treatment of Hashimoto's thyroiditis coexisting with papillary thyroid carcinoma. Adv Ther. 2007;24:510–516. doi: 10.1007/BF02848773. [DOI] [PubMed] [Google Scholar]
- 38.Hall FT, Freeman JL, Asa SL, et al. Intratumoral lymphatics and lymph node metastases in papillary thyroid carcinoma. Arch Otolaryngol Head Neck Surg. 2003;129:716–719. doi: 10.1001/archotol.129.7.716. [DOI] [PubMed] [Google Scholar]
- 39.Oh CM, Park S, Lee JY, et al. Increased prevalence of chronic lymphocytic thyroiditis in Korean patients with papillary thyroid cancer. PLoS One. 2014;9:e99054–e99054. doi: 10.1371/journal.pone.0099054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kwak HY, Chae BJ, Eom YH, et al. Does papillary thyroid carcinoma have a better prognosis with or without Hashimoto thyroiditis? Int J Clin Oncol. 2015;20:463–473. doi: 10.1007/s10147-014-0754-7. [DOI] [PubMed] [Google Scholar]


