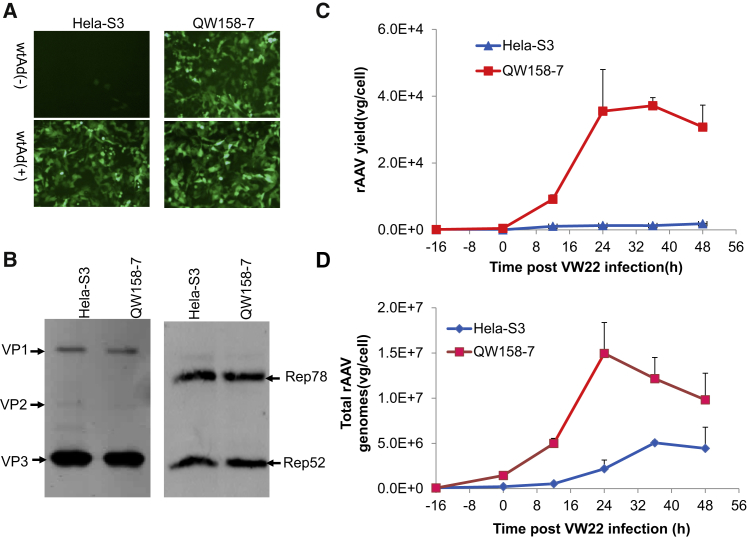
Figure 2.
Establishing an Optimized Suspension Cell Line for rAAV Vector Production
HeLa-S3 cells were co-transfected with pCMV-E1a/E1b and pCI-neo (100:1), and the best cell line supporting rAAV production (QW158-7) was selected, identified, and used for rAAV packaging. (A) Adherent cultured QW158-7 cells complemented VW22 for rAAV vector production in the absence of wtAd. Both HeLa-S3 and QW158-7 cells were tested for rAAV vector packaging with or without wtAd infection. The rAAV vectors with EGFP as reporter gene were collected and assayed at 36 hr post-VW22-infection. After three rounds of freezing/thaw and inactivation treatment by heating at 56°C for 1 hr, an aliquot of final vectors were used for comparison of rAAV yield using GM16095 cells. The EGFP+ cells were observed and photographed at 48 hr post-infection. (B) Western blot analysis of Rep and Cap proteins expressed in QW158-7 and HeLa-S3 cells. Cell lysates were harvested and analyzed 24 hr post-VW22-infection. (C) A time course of rAAV vector packaging in 100 mL suspension cultured QW158-7 cells. rAAV vectors were harvested and assayed at various time points post-VW22-infection. HeLa-S3 cells were used as control in the absence of wtAd infection. (D) Comparison of Ad/AAV replication property in QW158-7 cells and HeLa-S3 in the absence of wtAd. In all experiments, Ad/AAV-CMV-EGFP hybrid vectors (MOI = 50) were infected into cells 16 hr before adding VW22 (MOI = 4). The titer of rAAV vector genomes and total rAAV genomes were measured by qPCR as mentioned in the Materials and Methods. Bars represent the means of three independent experiments.